Prof.-Mishna Dafna Weiss from the Faculty of Biomedical Engineering at the Technion developed a method for estimating the metastatic potential of cancerous tumors. 90% of cancer deaths are due to metastases, so their early detection improves the patient's chances of survival
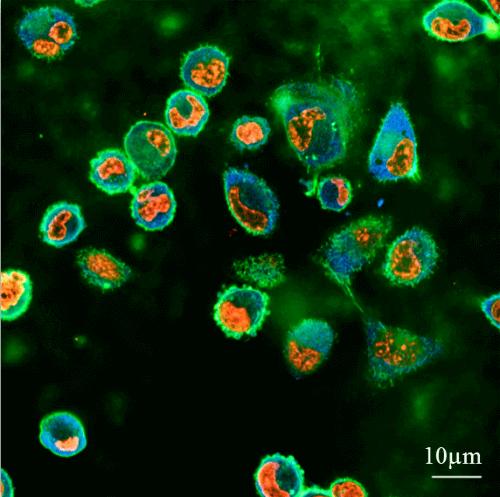
A research approach developed at the Technion will enable early and rapid prediction of the formation of cancer metastases. This information will allow doctors to treat these metastases already in the early stages of their formation, thus improving the patient's chances of survival.
The aforementioned approach was presented for the first time in 2013 and its development has continued since then in several directions, presented in three articles recently published by Associate Professor Dafna Weiss from the Faculty of Biomedical Engineering at the Technion. Prof. Mishna Weiss, head of the Laboratory for Mechanobiology of Cancer and Wounds, investigates the mechanical forces exerted on body tissues by metastatic cells - cells with a high metastatic potential. The research was conducted using synthetic gel surfaces that were produced in the laboratory of Prof. Mishna Weiss and whose stiffness simulates soft tissues in the body. The goal: to quantify the metastatic potential of cancer cells. In laboratory experiments, the forces that actuate such cells on the same surfaces to be pushed into them are tested.
In contrast to the treatment of primary tumors, which is currently done with high efficiency, the treatment of cancer metastases is complex and challenging. These metastases are sent to healthy organs through the lymphatic and vascular systems, and are difficult to detect in their initial stages of development. When they are detected, usually at a stage when they are already large and spread, medically dealing with them is very complicated. This is why cancer metastases are responsible for about 90% of cancer deaths.
In recent decades, various methods have been developed to identify the metastatic potential of cells, mainly based on genetic and biological markers. The disadvantage of these measurements is that they are expensive, take a long time and are not applicable in cancers such as pancreatic cancer, which have not yet been characterized by the markers that indicate them. In fact, so far no efficient, accurate and sufficiently general method has been presented to quantify the metastatic potential essential for predicting the formation of metastases.
In the researches of Associate Prof. Weiss, it was found that changes in the cell's structure and its ability to exert mechanical force may provide this vital information in a precise quantitative way. This method, which does not depend on the specific genetics of the tumor, allows a quick measurement (within a few hours) and personalized to the patient.
The synthetic gel surfaces developed by Prof. Mishna Weiss are similar in their level of difficulty to soft tissues and thus make it possible to study the conditions under which the cells exert force on the tissue into which they are trying to penetrate. This method makes it possible to quantify the amount of force they exert and the difference in the behavior of different types of cells. "The cancer cell strives to penetrate normal tissues and take over areas within," explains Associate Professor Weiss, "therefore, during evolution, cancer cells have developed structural flexibility that allows them to soften or harden in order to push through narrow areas."
Based on the properties of the healthy tissue and its structure, the cancer cells change their properties in aspects such as shape, internal structure and structural stiffness. "It is interesting to note that under certain conditions the secret of cancer cells is not hardness but rather softness - the cancer cell is softer and more flexible than a healthy cell, and the metastatic cell is even softer and more flexible. Cancer cells adapt to the environment quickly, and our method is based on identifying the changes that occur in them."
Three articles
The first article Out of the three, it was published in the journal Biomechanics and Modeling in Mechanobiology and is based on the research work of the master Sonobola Masalha. This study focused on cells that anchor themselves to their environment but do not attempt to penetrate the tissue. Masalha discovered a difference between benign cells and breast cancer cells, which exert more force on the tissue even though they do not try to penetrate it. These cells, although not yet invasive at that stage, influence the surrounding cells and can improve their ability to penetrate. The phenomenon of synergy between neighboring cells is demonstrated in another article.
The second article Published in the journal Tissue Engineering together with postdoctoral student Marta Alvarez-Elizondo. This study focuses on the relationship between cell migration ability and the mechanical permeability measured in the laboratory on the gels. The main findings: speed matters. Cells belonging to subpopulations characterized by a more developed ability to move are the ones that exert more force in an attempt to penetrate into the tissue. In the study it was found that the test method developed in the laboratory of Prof. Mishna Weiss is much more effective than the conventional methods for testing these properties, and provides a diagnosis of the cell properties within a few hours.
In the third article, published in the journal Annals of Biomedical Engineering, a model was built that more accurately simulates the processes occurring in the body. In the study, which was led by doctoral student Yulia Merhar, it was found that cells become more invasive when they move in groups or are in spatial proximity, and the explanation is simple: cells working together exert joint pressure on the tissue and thus increase their chances of penetrating it. According to Associate Professor Weiss, "With this discovery, we intend to go further and develop a rapid and quantitative prediction of the formation of metastases based on that group movement of cells."

Cancer cells, it turns out, interact differently with the tissue. Not only is the vertical force they exert on it stronger, but also the adhesion prior to the penetration attempt is performed with greater force while increasing cell motility. "The cancer cell remains round, with a small contact area, while benign cells elongate and increase the contact area with the tissue. It can be said that the benign cells are only busy with adhesion and function while the cell with the metastatic potential directs itself to change its environment and penetrate into the tissue. For this purpose, the metastatic cell organizes itself in a very different way in terms of morphology and communicates in a different mechanical way with other cells in its environment and with its environment. These are the clues that may help us in early and fast detection of these cells based on their mechanical properties. These abilities are of course caused by genetic changes, but with the approach we have developed there is no need for information about these changes."
These days, based on the approvals of the Helsinki Committee received already in 2015, Associate Professor Weiss is starting to check the findings on real tumors that were removed from the bodies of breast cancer, pancreatic cancer and stomach cancer patients as well as Ewing's sarcoma type tumors typical of children and youth. Associate Professor Weiss clarifies that the research is based on "leftovers" of tumor tissue that are not needed, even in terms of the pathological examination. "According to the initial findings, it seems that we really manage to identify the metastatic cells in these tissues based on their mechanical properties. Our applied goal is to develop a system that will allow the medical team to check, already during the biopsy or surgery, the likelihood of metastases of the tumor in other organs and to assess which organs are concerned. As mentioned, this is a very quick test, so that within two to three hours the doctors will be able to assess the metastatic potential of the tumor and adjust the treatment to this data."
Associate Professor Dafna Weiss completed her three degrees at the Faculty of Chemical Engineering at the Technion. She then went on to do a post-doctorate in the Department of Pathology at the School of Medicine at the University of California, Los Angeles (UCLA), USA. In her post-doctoral research, which was funded by NASA due to its implications for the fields of biology and medicine in space conditions, she began to engage in the subject she is currently engaged in: cell mechanics, with an emphasis on the behavior of cancer cells. She is included in the list of the 50 most influential women in Israel for 2015, published in "Lady Globes", due to her discoveries in the field of metastatic tumor diagnosis, which constitute "a breakthrough that will save lives in the future."

2 תגובות
In the broad sense, the word "evolution" is used in modern times to describe any process of change and development.
I don't understand the sentence: "explains Prof. Mishna Weiss, "therefore the cancer cells developed flexibility during evolution"
How can it be that cancer cells developed during evolution?
After all, a cancer cell kills its host and does not multiply, does not infect and does not move to another host, and does not express itself later in evolution (like a bacterium for example) and therefore a cancer cell cannot have descendants that undergo evolution.
Therefore, it is not clear to me how cancer cells can change during evolution?