In the future, this discovery will make it possible to better understand the mode of action of chemotherapy drugs that hitch a ride on hCTR1 and penetrate the cell in a similar way, and to improve the chemotherapy treatment of cancer. The study was published today in the scientific journal PNAS.

Prof. Nir Ben-Tal, PhD student Maya Shoshan, and Master's student Yariv Barkan in the Department of Biochemistry and Molecular Biology at Tel Aviv University in collaboration with Prof. Turken Haliloglu from Istanbul calculated the detailed structure and mode of action of a protein called hCTR1 (human copper transporter 1), one of the proteins located in the membrane The cell (the fatty envelope that surrounds each of our body's cells and delimits it from the environment). hCTR1 is responsible for transferring copper ions into the cell. In addition to this role, which evolution gave it, it turned out that it also transports the active chemotherapy substance cisplatin into the cell.
According to Prof. Ben-Tal, the current research is a first step on the way to understanding how the chemotherapy drug penetrates the cell and may help in the future to design better drugs. The results of the study were published today in the scientific journal PNAS (Proceedings of the American National Academy of Sciences).
About 30% of the proteins in our body make up the cell membrane (and help to maintain a proper substance balance inside the cell). Compared to proteins found inside the cell, it is very difficult to determine the three-dimensional shape and the movement ability of the cell membrane proteins. Until the current study, the details about hCTR1 were known with a very low resolution, and it was not known at all how it moves, and therefore in what way it introduces the copper ions into the cell.

Copper ions, in low concentrations, are essential for the cell and are required for the action of various enzymes that participate in vital processes such as blood clotting, protection against oxidants and cellular respiration. Damage to the activity of the copper-binding enzymes may therefore cause serious diseases such as hemophilia, anemia, diabetes and heart disease. However, the copper ion in itself is highly reactive, and tends to rapidly generate free radicals. As a result, during evolution, complex mechanisms were developed to transfer the ion from "hand to hand" in the chain, with the different hands being proteins of different types. hCTR1 is one of the proteins in the chain.
"The goal of our research was to reveal new details about the structure and movement of hCTR1 through computational models, to locate certain amino acids responsible for the protein's activity and to propose a mechanism for its action." Prof. Ben-Tal explains.
During the study, the researchers compared the protein between different animals and discovered which elements were preserved throughout evolution between the different species and which were not. Among the preserved elements, the researchers located those responsible for transferring the copper ions into the cell in all animals and therefore also in humans.
"In the first step, we used the existing low-resolution structure of the protein and the evolutionary information in order to predict the high-resolution structure."
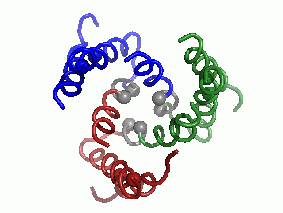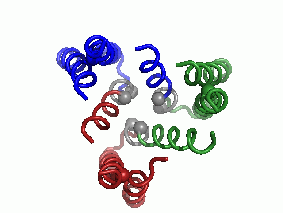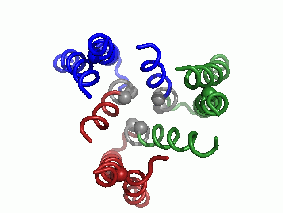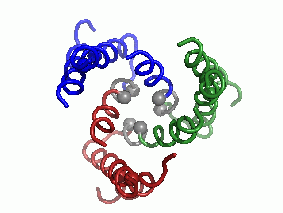
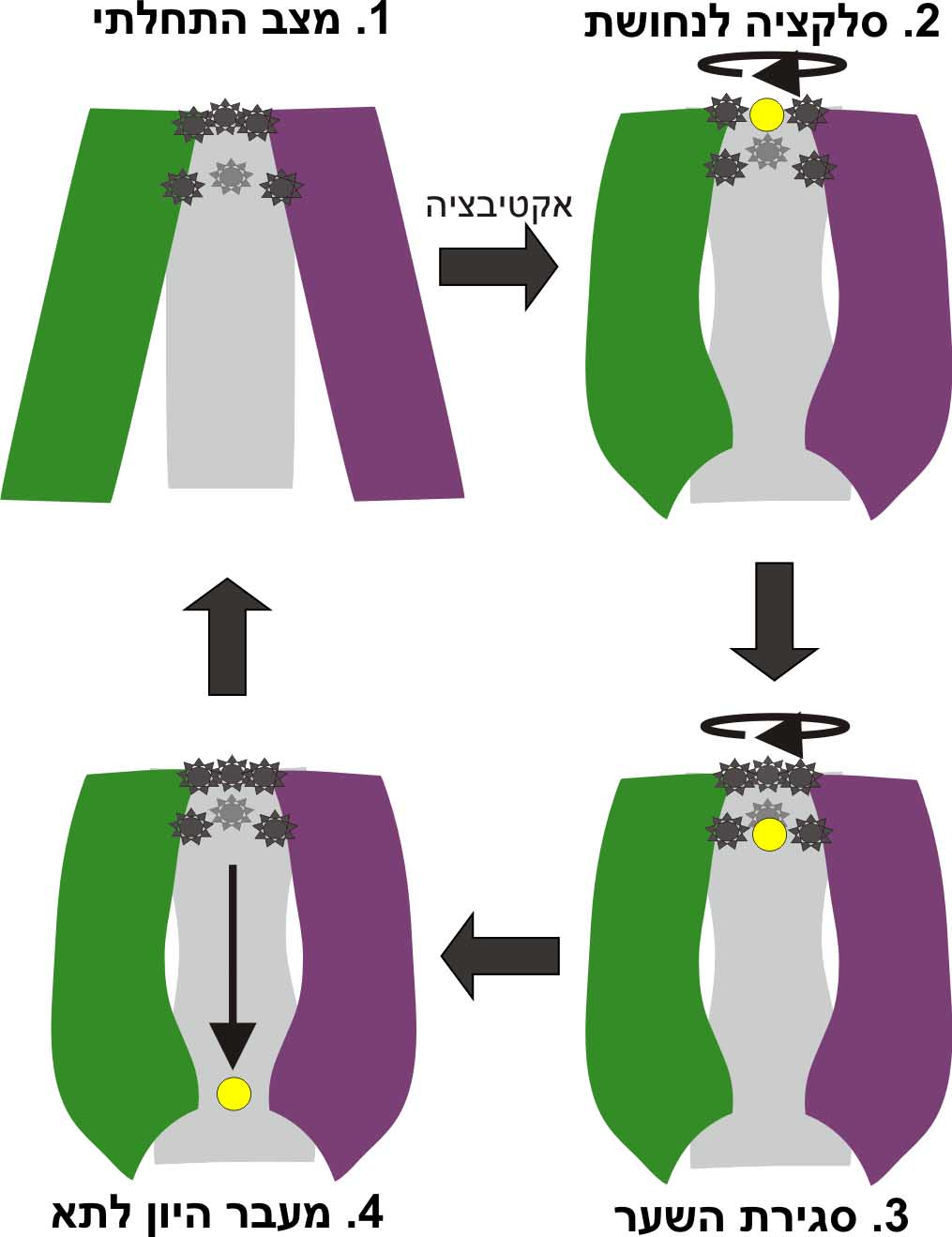
"Every complicated movement can be described as a sum of simple rocking movements called 'oscillating bikes'. In a second step, after predicting the protein's structure, we investigated, in a computational way, its modes of oscillation. We discovered several main modes of oscillation that make up the overall movement of the protein. (The main mode of oscillation is shown in the video clip - picture 1).
The last stage of the research is the most important: what can be learned from predicting the molecular structure and movement of hCTR1 about its activity? In this case, the calculations helped us to propose how hCTR1 transports copper ions into the cell while protecting against the harmful radical activity of the ion, i.e. what is the mechanism of action at the molecular level (picture 2).
"The process is carried out in the following way: after a single ion undergoes the selection, the structure of the protein changes so that the entrance to the channel is blocked, and additional ions cannot enter. The ion that passed enters the cell, and God forbid returns. The introduction of the ions one-by-one through the channel allows a tight control of their entry into the cell. The mechanism demonstrates how the cell manages to absorb ions that are essential for various processes while controlling their destructive potential." Shushan explains.
"The research is another step on the way to understanding the cellular mechanism for transporting the copper ion." Prof. Ben-Tal explains, "Also, it reveals the uniqueness of hCTR1, both in terms of the general architecture and in terms of the transmission mechanism, compared to membrane proteins that transport other ions. Since, as mentioned, hCTR1 also transports cisplatin, a substance used in chemotherapy treatments, this understanding also has implications for the study of the transmission mechanism of cisplatin; The knowledge gained may lead to an improvement in chemotherapy treatment.

One response
We have excellent researchers in the country