The journal SCIENCE chose, in addition toBreakthrough of the year, a number of noteworthy events in several scientific fields. One of these fields is the field of genetics.

Genetic variation is associated with over 60,000 human diseases, nearly 35,000 of which are caused by small errors in DNA - a change of one base at a certain point in the genome. This year, researchers announced a significant improvement in an emerging technique, called base editing, for correcting such point mutations not only in DNA, but also in RNA.
The pioneer of the field, David Liu, a chemist at Harvard University, used the CRISPR base-editing system, which was first demonstrated as a powerful laboratory tool in 2012. CRISPR excels at cutting DNA at specific locations, detecting errors and turning off genes, but has only partial success in correcting errors caused by point mutations , that one of the four DNA bases ie A, C, T and G — was replaced by another. Liu's group modified the existing options in CRISPR to create this base editor that would not cut the DNA at the target location but replace one chemical base with another. Last year the researchers succeeded in replacing C with T and this year they replaced a wrong G in the most common mutation. Notably, a separate team led by Zhang Fang of the nearby Broad Institute in Cambridge, Massachusetts, demonstrated base editing in which they replaced G and A in RNA.
This year, the researchers demonstrated the power of the ability to edit bases by correcting a disease-causing mutation in human embryos. They never intended to grow the embryos, the repair was not always successful, but the achievement proved that editing the base has enormous potential. In their view, CRISPR is a gift that continues to yield breakthroughs.
a cure for cancer adapted toDNA of the cancer and not to the organ from which it grew
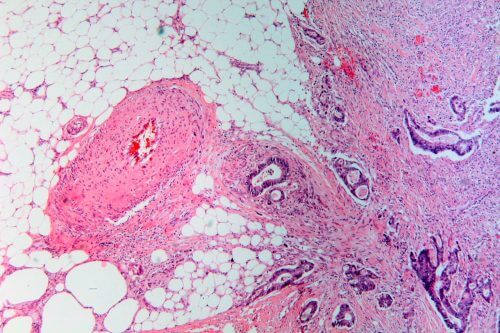
In May, the US Food and Drug Administration (FDA) gave the green light to the product pembrolizumab manufactured by Merck, under the brand name Keytruda. The drug, which has already been approved for the treatment of melanoma and a handful of other types of cancer, is now given in any case of an advanced solid tumor in children or adults, under one condition: the cancer cells must carry a defect with the strange name of mismatch repair deficiency. DNA repair Type MMR) This means that if the cancer cells are found in the pancreas, colon, thyroid gland or any of a dozen other tissues - they must carry the DNA mutation that the drug corrects.
The FDA's approval implies a big change for the field because it shows that tumors originating from different organs have much in common. But turning this knowledge into treatment was not easy. A breakthrough came in 2015, when doctors at Johns Hopkins University in Baltimore, Maryland, led by Luis Diaz of the Memorial Sloan Kettering Cancer Center in New York, studied pembrolizumab in colon cancer patients. They noticed something striking: in eight out of 13 people suffering from mismatch repair deficiency, the tumors shrunk, and in another four they kept their size but stopped growing when the drug was administered, the drug formed an "inhibiting immune barrier" that allows the immune system to fight cancer. 25 other colon cancer patients without the defect did not respond to treatment. The doctors' theory is that cells containing the barrier do accumulate hundreds of mutations, but the immune system recognizes these cells as foreign easily and kills them.
A study published in June by Diaz Lee, from Johns Hopkins University, and other studies together extended the experiment to 86 patients suffering from 12 different types of cancer, all of whom had mismatch repair deficiency. 53% responded to the drug. Based, among other things, on this study, the FDA approved a drug that oncologists hope will be the first of many drugs in the new strategy against cancer.
A victory for gene therapy
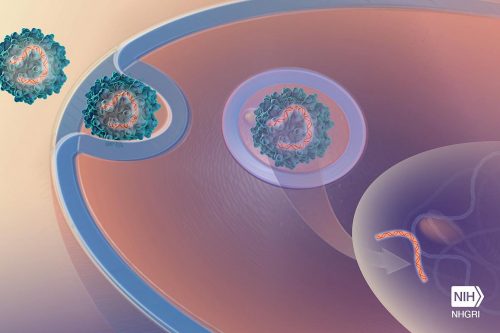
Success in a dramatic small clinical trial looms large in the field of gene therapy this year. Researchers have reported that they have saved the lives of babies born with an inherited muscular dystrophy by adding a gene to add neurons to their spines. If the babies are not treated, they may die by the age of two.
The experiment also marks a more significant milestone, because the researchers passed the new gene through the membrane that protects the brain and spinal cord using pathogens and toxins. This experiment could open the door to gene therapy of other neurodegenerative diseases.
The key was a harmless virus, called AAV, widely used in gene therapy in laboratory mice. In 2009, research groups in France and at Children's Hospital in Columbus, Ohio, discovered that a strain of the virus called AAV9 injected intravenously into newborn mice could spread through the brain and spinal cord.
Now, researchers have shown that gene therapy using intravenous AAV9 can stop spinal muscular atrophy type 1 (SMA1), the most common genetic cause of death in infants. In newborns with SMA1, the protein needed for the movement of neurons in the spinal column is missing; The muscles eventually atrophy and the newborns cannot breathe. In November, members of the research group and the AveXis company reported that each of the 12 babies who received a high dose of AAV9 carrying the gene for the missing protein - could speak, eat and sit by themselves, at least for a short time. One of the girls even managed to walk fast and be an active girl. The new drug achieved similar results, but it must be injected into the spine every few months.
Researchers are now using AAV9 that carries other genes to treat children with severe inherited brain disorders. In the past, researchers had to drill holes in the skull to deliver gene therapy to these children, and the treatment was also ineffective.
This year, two cancer treatments were performed in which the patient's immune system was genetically engineered outside his body and it was transplanted back into him. These treatments are the first genetic treatments to reach the American market. On December 19, the Food and Drug Administration (FDA) approved the first gene therapy for a rare genetic disorder that causes blindness.
Summary of 2017 on the knowledge website:
- Neutron star merger - breakthrough of the year according to SCIENCE magazine
- Drought, hurricanes and Trump: 2017 summary in the environment
- 2017 in space: the chairman of the space agency is optimistic about the communication satellite program and the SpaceIL project
- 2017: SpaceX's incredible year
- 2017 in space - the end of the Cassini mission to Saturn

One response
A clear and beautiful article.