Scientists discover for the first time how toxins from bacteria, "superantigens", work and how short protein segments they have created can block them and thus save lives
The Hebrew University.
Infections from staphylococcal and streptococcal bacteria affect millions of people every year. They are a leading cause of sepsis and are responsible for many cases of pneumonia and postoperative infections. Their ability to cause infectious diseases stems from the production of many virulent factors, among which a group of 'superantigens' - toxins that accelerate the infectious process - plays a decisive role.
Several dozen species of superantigens are lethal to humans and are a decisive factor in sepsis and the development of toxic shock. Although the inflammatory immune response triggered by antigens is necessary to protect humans against viruses and bacteria, in the case of superantigens the excessive response triggers an "immune storm" - which can cause great damage to the body and lead to organ failure and even death.
Even with the application of current treatment approaches, these infections have high mortality rates and their treatment has become even more complex due to the emergence of multidrug-resistant bacterial strains. Due to the increasing urgency and aggravation of these situations, the depletion of the development pipeline of new antibiotics and the spread of bacterial resistance to the variety of drugs, the classic approach of one drug for each invader is already outdated and less effective.
Now, in a scientific work that is a landmark at the culmination of 20 years of research, scientists at the Hebrew University of Jerusalem describe an innovative therapeutic approach capable of preventing fatal immune reactions, bypassing the bacteria's resistance to antibiotics and targeting the individual himself. The new approach has important implications for medicine: it is effective and wide-ranging and is not affected by the bacteria's resistance to antibiotics.
In an article published in the prestigious journal PNAS (link), the researchers discover for the first time the mechanism of action of the superantigenic toxins, and how antagonists they designed are able to block this action and thus save lives.

Prof. Raymond Kempfer, from the Dr. Philip Marcus Chair in Molecular Biology and Cancer Research at the Israel-Canada Medical Research Institute in the Faculty of Medicine of the Hebrew University, led the research and explains the change in the new therapeutic approach in light of the discovery of the mechanism in which the super antigens activate the inflammatory response even more :
"Instead of focusing the fight against the invading bacteria, which can undergo mutations and develop resistance to antibiotics, we direct the medicinal activity to the person, so that even before the invader can cause a serious illness, it must pass through the 'bottleneck' of the receptors which we are able to effectively block. The advantage of this is that the drugs remain effective even against strains of bacteria that are resistant to antibiotics."
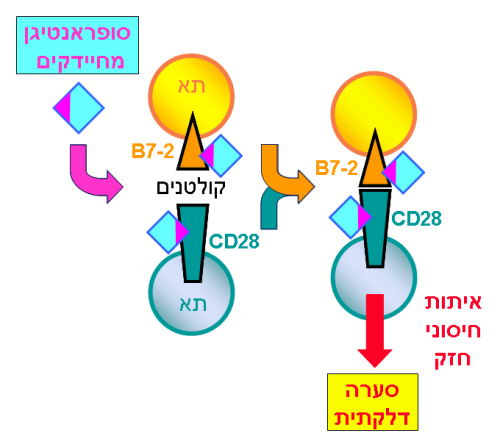
In a normal immune response as a result of exposure to an antigen, immune cells adhere through receptors placed on their surface, called "co-stimulatory" receptors. Surprisingly, the superantigen catalyzes the binding between the main co-stimulatory receptors, B7-2 and CD28, and it turns out that this catalysis is critical to the toxin's ability to trigger an immune storm. The creation of the co-stimulatory axis was already known to be important for the development of a full immune response, but the new study highlights the importance of this axis as a major "bottleneck".
The research team had already shown in previous studies that the superantigen must bind to the CD28 receptor to cause damage, but the mechanism behind this requirement remains unknown. Now, the researchers show that in order to catalyze the binding between the CD28 and B7-2 receptors, the superantigen molecules must bind directly to these two receptors. By directly binding not only to CD28 but also to B7-2, superantigens significantly increase the binding between these receptors, increase the creation of the co-stimulatory axis and thus induce T-cell hyperactivity and thus trigger a pathological immune storm.
The discovery of this mechanism led the researchers to design new peptides - pieces of protein in the human B7-2 receptor - capable of effectively blocking the binding between the superantigen and its target sites in the co-stimulatory receptors, thus protecting against lethal shock, as the researchers showed in animals. Each peptide mimics a small fragment of a site on B7-2 that the superantigen must bind to. Such a peptide acts as a mimic of the intact receptor that binds the superantigen toxin. In this way, the peptide blocks the access of the toxin to the receptor and prevents its toxicity.
"The strategy of using peptides that mimic regions of a receptor in the immune system in order to curb the excessive inflammatory response released by superantigenic toxins, is a strategy aimed at the host - the person himself. We have shown that this approach is broadly useful against the diverse family of superantigens," said Prof. Kaempfer.
Dr. Alan S. Cross, a professor of medicine at the University of Maryland School of Medicine in the US, who did not take part in the research, responded to the research discovery: "This is a conceptual breakthrough in our understanding of the mechanisms of infection and disease caused by an excessive immune response. It may have implications for novel approaches to the treatment of other diseases characterized by a "cytokine storm". This new approach, to act against the strong response of the host that causes the disease instead of attacking the microbial pathogen, is gaining broad support in the medical community."

One response
"In an article published in the prestigious journal PNAS (link)" -?