Institute scientists are looking for the genes responsible for brain development

During the development process of the fetus in the womb, a mass of several cells becomes a complete and functioning animal. One of the fascinating aspects of this process takes place in the brain: billions of cells are created, migrate to their exact location, send out extensions that connect them with other cells, and ultimately create the command and control machine that allows us to move our organs, see, think and feel. It is the migration of nerve cells from the center of the brain to the outside that is responsible for the layered structure of the cerebral cortex, and the ability of nerve signals to move between the different layers, and between them and the structures in the depths of the brain. During the migration, the cells mature and undergo internal and external changes: the "polygonal" nerve cell (multipolar) takes on an elongated and asymmetrical (bipolar) shape, with a defined direction and characteristic extensions.
The change of shape is a central part of many events related to the development of the central nervous system. The migration and the changes that take place during it are made possible thanks to a long series of auxiliary means - starting with special cells that create "rails" on which the nerve cells move, to intracellular mechanisms whose purpose is to pull the cell - and especially the heavy nucleus - during the migration. For this purpose, there are changes in the proteins that form the intracellular skeleton, which supports the cell structure: the rigid and stable structure is replaced by a flexible and dynamic skeleton that allows movement. The control of all these processes is done by genes, which precisely control the timing and location of the various events. Disruptions in the control mechanisms may cause diseases such as schizophrenia, degenerative brain diseases, and "lissencephaly", which is characterized by a brain without wrinkles and folds, and causes severe retardation and death at a young age.
Prof. Orly Reiner, from the Department of Molecular Genetics at the Weizmann Institute of Science, is looking for the genes responsible for brain development. Her search for suitable candidates recently led her to focus on the Par1 gene, which encodes the information needed for the construction of an enzyme that plays a role in determining the directionality of nerve cells, and also regulates the changes in the stiffness of the skeleton of these cells (it causes the detachment of proteins attached to the skeleton, and as a result increases its flexibility) . The involvement of the enzyme Par1 in these events raised the suspicion that it actively controls the migration of nerve cells during embryonic development. A study, the results of which were recently published in the scientific journal Journal of Neuroscience, shows that the enzyme does indeed play a central role in the control of migration, and that the presence of an appropriate amount of it is a critical matter - both an excess of Par1 and a lack of it are harmful to brain development - albeit in different ways.
In the first phase of the research, conducted by Dr. Tamar Sapir together with research students Sivan Spoznik, Danit Finkelstein and Anat Shmueli, and laboratory technician Talia Levy, the researchers asked to examine what happens when the activity of Par1 is prevented in the developing fetal brain. For this purpose, they used a method developed by Dr. Sapir: they injected short RNA molecules (siRNA) into the cerebral cortex of the mouse embryos. After that, an electric current was passed which allows the penetration of the molecules into the nerve cells adjacent to the space. The RNA molecules bind to the Par1 gene and interfere with its translation process into an active protein. In fact, they prevent his activity. In addition to them, a gene is injected that produces a fluorescent substance, which makes it possible to identify the cells in which the molecules have been absorbed, and to track their movement. The process is carried out on the 14th day of pregnancy, when migration reaches its peak. After four days, during which fetal development continues normally in the womb, the brains of the mice are examined.
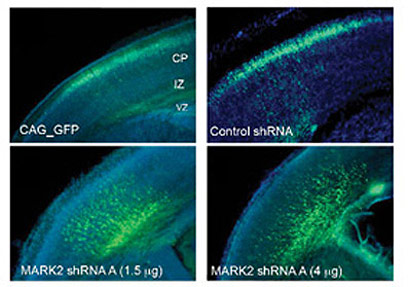
The researchers discovered that damage to the Par1 gene inhibits the migration of nerve cells: while in the control mice the marked cells reached the outer layer of the cerebral cortex, in mice in which the gene was silenced the nerve cells "stuck" halfway. The stopping of the cells occurred in a specific location - where the changes in the structure of the nerve cells occur, and they acquire their directionality. Later, the researchers tried to restore to the nerve cells the ability to migrate by inserting the Par1 gene using genetic engineering methods. The introduction of the gene did succeed in rescuing the stuck nerve cells, but only when it was given in a precise dose - too large concentrations caused the formation of rounded cells, without extensions, which are characteristic of nerve cells.
In another series of experiments, the researchers tested how exactly Par1 affects the ability to migrate. It turned out that damage to the gene increases the stability and rigidity of the intracellular skeleton, which makes it difficult for the cell to move. In addition, the movement of the centrosome is affected - a structure that is part of the intracellular skeleton, whose role is to "pull" the continuously migrating cell forward. It seems that as a result of damage to the Par1 gene, the movement of the centrosome became slow, and lacks a defined direction.
"The migration and the structural change are interwoven processes," says Prof. Rainer. "Our findings indicate that Par1 is the link between the two processes. It does this through double control - on the directionality of the cell, and on the dynamics of the intracellular skeleton." Prof. Rainer hopes that a thorough understanding of the factors that regulate the migration of nerve cells, and the events that accompany it, will help in the future to deal with diseases caused by improper organization of the brain structure. Such information will be essential, among other things, for the development of future healing methods based on the injection of healthy nerve cells into the patient's brain, and the precise targeting of the cells to the damaged area.

3 תגובות
I don't care about the gayety
The understanding:
What exactly do you understand?
Can you point to a previous article where what is said here already appears?
Lol
What will happen?
What will be the end with the reconstructions of these experiments?
There is nothing new here.
Enough to repeat experiments carried out years ago!
The Weizmann Institute please come to your senses, kick these females out of the research facilities and start moving forward!