Stem cells are like the fountain of youth - a life elixir that will one day repair a tired and damaged heart muscle, or put an end to a degenerative disease. But the reality is still far from the expectations. A study recently carried out by Prof. David Gavol and his research partners from the Department of Molecular Biology of the Cell at the Weizmann Institute of Science may, perhaps, in the future, offer a solution to some of the problems associated with the medical use of stem cells.

Many studies in stem cells are carried out in embryonic stem cells - the initial cells of the developing embryo. Embryonic stem cells are able to differentiate in any direction and become any type of cell in the body. Thanks to this unique feature, they are considered not only an ideal research tool, but also - at least theoretically - an ideal medicine, which may form the basis for the treatment of a long list of diseases, in particular those related to aging
and cell degeneration. The problem is that the only source of these cells is human embryos, which places ethical restrictions on their use.
In 2006, the Japanese scientist Shinya Yamanaka proposed a way to solve the problem, causing a stir in the world of science: his research showed that it is possible to "reprogram" mature skin cells, so that they become embryonic stem cell-like cells. These cells are not exactly identical to embryonic stem cells, but they have the ability to differentiate into any cell type. The process of "reprogramming" the cells was surprisingly simple: it turned out that it was enough to insert four genes into the DNA of the skin cell to turn back the clock, and change cells whose fate was determined a long time ago. The new cells were named iPS (induced pluripotent stem.

"The importance of this discovery," says Prof. Gavol, "was not only in its possible future biomedical applications, but also in the fact that it overturned the old assumption that cell differentiation is a one-way process that can never be reversed."
In reality, although our cells are usually "locked" in a closed pathway, in fact the cells of all our tissues - from muscles to skin - contain all the genetic tools needed to create all types of cells. In order to reprogram them, we need, in a sense, to find out how to activate certain genes and stop the activity of other genes, thus returning to the state of the embryonic stem cell. It turned out that the four genes needed for reprogramming belong to a small group of genes that activates other genes in the embryonic stem cells. After cell differentiation, these genes are "silenced".
Despite the breakthrough, the new iPS cells cannot be used as a medical device. The problem is that the four genes inserted into them for the purpose of reprogramming remain in their place within the DNA, and continue to operate - and activate additional genes - even at the stage when they are supposed to be silenced. Such activity may cause cancerous processes and harmful mutations. "In fact," says Prof. Gavol, "any introduction of DNA into the genome is dangerous, and may cause unexpected results."

For this reason, since the first iPS cells were created, many scientists are looking for a way to avoid the risk. Among other things, complex methods were proposed for removing the pieces of DNA after they had finished their work. In another approach, the insertion of the genes is replaced by the direct insertion of the gene products - proteins. But both approaches are still problematic. Prof. Gavol, along with his colleagues, Dr. Edward Yaakovov and Prof. Shmuel (Shapilka) Rosenblatt from Tel Aviv University and Prof. Gideon Rafi from the Sheba Sheba Medical Center in Tel Hashomer and Tel Aviv University, chose a middle way. Their method is based on the mediating factor between DNA and protein: messenger RNA molecules (mRNA), which lead the program for producing proteins from the cell nucleus to the protein factories - the ribosomes. The RNA molecules break down after a short time - something that can prevent the problems arising from foreign genetic material remaining in the cell. An even more important feature of RNA is that it does not integrate into the genome, and therefore does not pose a danger for the formation of mutations. On the other hand, the proteins it encodes are created, folded and processed by the machinery of the host cell, so they are expected to function properly and without interference within it. The scientists created messenger RNA of the four genes required for reprogramming in vitro, and injected it into adult cells grown in culture. They repeated the insertion several times, and after a week the mature cells expressed genes characteristic of embryonic stem cell activity. When the cells were transferred to another growth surface, the cells - originating from connective tissue - began to grow long arms characteristic of nerve cells. The researchers called these cells iPS - RiPS created using RNA. Additional experiments are required to check if the new cells are indeed endowed with all the properties of iPS cells.
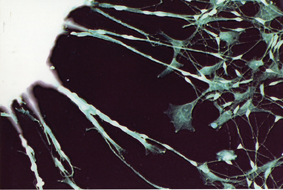
Prof. Gavol: "At this stage we have proven that it is possible to carry out reprogramming using messenger RNA, and that it serves as an effective substitute for DNA. It seems that methods based on RNA will take a very important part in cell programming." Several research groups, including those hoping to develop biomedical applications, have already shown interest in the new method. Prof. Gavol: "Stem cells reprogrammed using RNA may promote the development of personalized medicine by cells. In the future, it will be possible to create iPS cells by reprogramming the cells of an adult person, and later transform these stem cells into the desired cell type - also using messenger RNA of the genes responsible for the desired differentiation, such as muscle, nerve, etc. These cells could be used to treat many diseases, including those that cannot be cured today."

8 תגובות
I would love to hear a professional opinion about the function of stem cells in the cosmetics industry. How well it really works. Thanks in advance.
Greetings
Is there compassionate stem cell-infused treatment for Huntington's?
An orphan disease that is hardly treated.
Thank you in advance
Thank you very much R.H.
Daniel,
Your description is not accurate. The purpose of the heating is to open the double strand into two single strands and not "cross the DNA"
You add the primers to the reaction. The primers are 2 short single-stranded DNA sequences (about 20 bases) that match and border the segment you want to amplify.
The chemical reaction that binds the primers is simply the usual base pairing of AT and GC. Since the primers are short, their binding temperature is higher than that of the original single strand, therefore at the annealing temperature (about 60C, according to the sequence of the primers) they will bind, but the second strand of the DNA will not bind. The polymerase joins and from the primers copies the template to which they are attached.
I hope the explanation is clear and if not just look at a diagram and it will be clear.
I would like to ask a question that is not necessarily related to the section presented. I would appreciate a detailed answer.
When you put any gene into a PCR device, the first step is to heat the device to about ninety degrees Celsius. To cross the given section in two. And from there the primer connects to that section (crossed). and from there the DNA polymerase molecule. Continues to build a matching sequence on top of the segment, thereby causing its duplication. And I would like to know where that primer comes from to the segment so that the molecule can continue. And I would like to know what the chemical reactions are that cause this.
So why can't the method also work in making other proteins in normal cells like those needed in genetic diseases like cystic fibrosis.
Or proteins whose lack leads to the creation of cancer and more.
Can anyone explain?
Nice, sounds really good