Scientists from several research institutions recently collaborated to try to find out whether the role of water is limited to being "that substance in which everything swims", or perhaps it takes a more active part, and examined what happens when water molecules meet an enzyme
Everyone knows that water is essential to life, but scientists who study the processes of life tend to ignore the presence of water - at best, they treat it as a liquid in which the "important things" float. The main reason for this is that the water molecules are tiny and very fast, while each protein molecule is a thousand times larger and slower than them. Therefore, the microscopic methods by which large biological molecules are observed are actually "blind" to the thousands of water molecules that are around them.
Scientists from a number of research institutions recently collaborated to try to find out whether the role of water amounts to being "that substance in which everything swims", or perhaps it takes a more active part, and examined what happens when water molecules meet an enzyme. The findings reveal that at least in one of the stages of the process, water plays an important role - it helps the enzyme bind to its target protein.
The enzyme studied by the scientists belongs to a family of proteins that has been studied for many years in the laboratory of Prof. Irit Sagi, from the Department of Biological Control at the Weizmann Institute of Science. This enzyme, as well as its family members, digest biological molecules. In fact, the enzyme family plays an essential role in a variety of biological actions, from cell movement to tissue remodeling, and it may also help the movement of cancer cells throughout the body.

In previous studies, Prof. Sagi developed innovative dynamic methods based on X-rays, and used them to create "movies" describing the activities of various important proteins. The current research was done by research student Moran Grossman from Prof. Sagi's group, in collaboration with Dr. Mathias Hayden and Dr. Benjamin Born (currently a post-doctoral researcher in Prof. Sagi's group), from Prof. Martina Haybanit's research group from the University of Rohr in Germany, and with Prof. Greg Fields from the Torrey Pines Institute for Molecular Research in Florida. The team combined Prof. Sagi's method with terahertz spectroscopy - based on short pulses of terahertz radiation - to reveal the dynamics of water molecules together with the enzyme. The unique combination of research methods allowed them to obtain data at the resolution level of single atoms, and in real time. The research findings were published in the scientific journal Nature Structural and Molecular Biology.
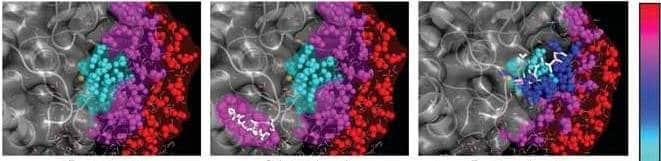
The enzyme studied by the scientists contains a metal ion (in this case – zinc) in the core of its active site. It is the metal ion that actually determines the total electric charge inside the core, while the enzyme performs its activity. Water is naturally attracted to such charged atoms, because it is itself a charged molecule: the side where the oxygen atom is located carries a weak negative charge, while the two hydrogen atoms, which form a kind of angle with the oxygen atom, and are on the other side, carry a weak positive charge (this polarity is also the reason Because water is liquid - and not gaseous - at room temperature: the molecules form electrical connections for a short time before they pass each other).
The team discovered that the nanoscopic molecular motions of the water in the active site, as well as other dynamic properties of these molecules, differed from the properties of the water molecules surrounding the enzyme or located further away from it. The exchange of bonds between the water molecules in the active site - in the presence of the metal ion - was very slow. As a result of the slowdown, the water became a viscous substance - more like honey than a flowing liquid. In the first stages of the enzyme's activity, the scientists were able to identify a direct connection between the changes that occur in the enzyme's configuration during its activity and changes in the movements of the water molecules around it. During the process, the water molecules with the slow binding left the active site, to make room for another protein - on which the target site of the enzyme is located. The researchers believe that this change in water movements is a general phenomenon, whose role is to help the enzymes make contact, in the correct configuration, with their target protein.
Prof. Sagi: "It turns out that this 'marriage' between the water and the enzyme is a very complex process. By combining structural-biophysical methods with protein engineering, we were able to advance our understanding of nature's plans."
Does the water play other roles in the activity of this enzyme? And how do they contribute to other biomolecular processes? For Prof. Sagi and Prof. Haibanit and the members of their research groups, this research is just the beginning of the road. The scientists believe that understanding the exact role that water plays in the activity of many biological molecules may be especially essential for the purpose of designing and manufacturing medicines - including some medicines that are developed in their laboratory.

One response
The way I see things now, we are very far from being able to understand what is really happening there.
When I say understand I mean being able to type it on a computer and get the same result.
Just so you understand what it is about, even a simulation of the interaction of the forces of one water molecule to the neighboring molecules is imprecise (an error of a few tens of percent in relation to the measurements). So who even talks about simulating complex systems the size of one cell (a 10 cubic micron cell will contain about a million millions of atoms).
In short, you can forget about it.