Scientists studying the precise activity of cells may receive assistance in their work thanks to a new method for labeling and imaging proteins inside living cells
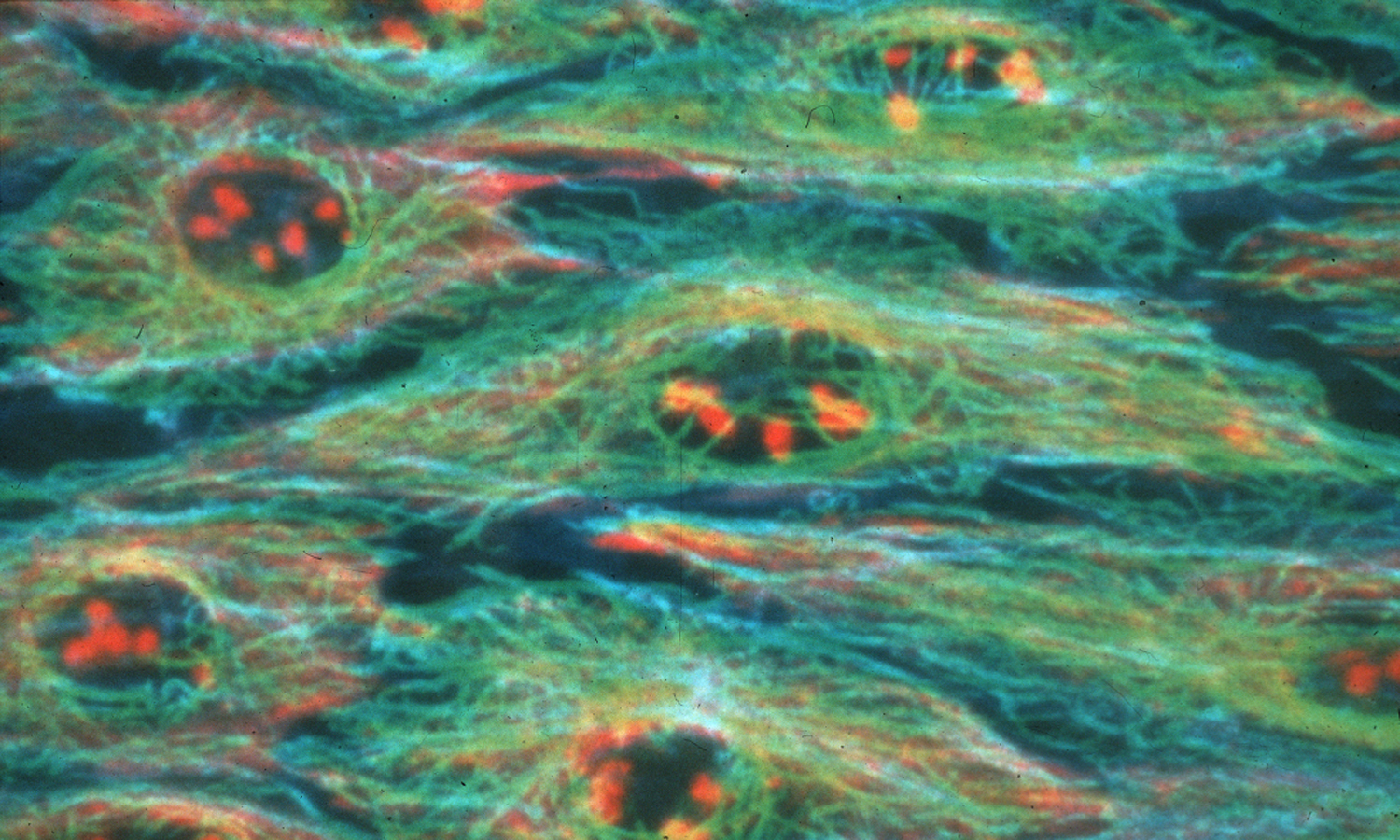
The new method, developed by a research team from the University of Illinois led by chemistry professor Lawrence Miller, provides the clearest and most dynamic view obtained so far of the interactions between proteins in cells using a unique microscope. The research findings are published in the scientific journal Proceedings of the National Academy of Sciences.
Knowing the location and timing of the activity of certain proteins in the cell is a key factor in understanding life processes at the molecular level.
In the new method, known as "luminescence resonance energy transfer", two proteins in the cell are labeled with luminescent beads of different colors, which absorb light in one color and emit it in another color. By obtaining several images of the cell, which contains these proteins, and their mathematical analysis, the researchers are able to obtain information regarding the exact location of the proteins and the nature of their mutual activity.
The researchers used a new type of luminous probe for the tagging, thanks to which it was possible to get the same information using fewer images. This fact simplifies the analysis and allows information to be processed five orders of magnitude faster than what is customary today. The resulting images themselves were fifty orders of magnitude higher in sensitivity than usual.
The researchers used a combined chemical-genetic approach to label the proteins that answered them. One of the proteins was genetically modified so that it could bind to a metallic conjugate of terbium (Tb). This coupling has an extremely long time interval between the moment of absorption and the moment of emission of the light. The second protein is genetically modified so that it can bind to a luminescent tag with a short emission duration. When the two proteins react with each other, their luminescent tags move closer to each other and produce a unique luminescent signal, which can be detected with a microscope.
The group of researchers modified a standard microscope to take advantage of the long lifetime of the terbium tags in the proteins. Light projection was used to initiate the light emission of the terbium and to detect it after the other luminescent forms within the cells had already become inactive (black and invisible), making it possible to remove unwanted background from the resulting image.
The new method "increases the sensitivity and makes the whole process faster," explains the lead researcher. "These factors increase the time separation of the experiment, so that the changes in protein activity can be seen in shorter time intervals, a situation that allows you to better understand biological phenomena."
The method required finding a reliable way to transfer the luminescent terbium detector through a living cell membrane without contaminating or damaging the cell itself. To this end, the researchers developed a way to take advantage of the cell's own pinocytosis process, a process in which the cells take in small amounts of the extracellular fluid around them.
"Using this innovative tool, we hope that cell biologists and others will be able to investigate and understand phenomena that have not been seen before, such as interactions that could not be seen in living cells in real time," the researcher notes. "We hope that the method will lead to obtaining information that will make it easier for us to understand how biological mechanisms work."
