The findings will lead to the establishment of power plants that will more successfully capture carbon dioxide and less of it will be released into the atmosphere
Chemists from UCLA University report on significant progress in reducing the emission of the greenhouse gas carbon dioxide in the journal "Science" in the 15.02.2008 issue.
The scientists demonstrated how they can successfully isolate and capture carbon dioxide, a greenhouse gas that contributes to global warming, rising sea levels and increasing ocean acidity. Their findings could lead to the establishment of power plants that will successfully and safely capture the carbon dioxide released into the air without the use of toxic substances. "We managed to overcome the technical challenge in the selective removal of carbon dioxide," says Omar M. Yagi and chemistry professor Christopher Foote, co-authors of the paper.
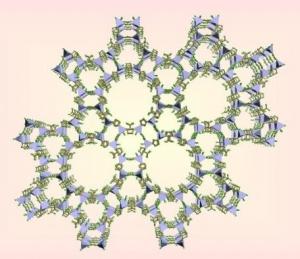
"We now have buildings that can be designed to selectively and precisely capture carbon dioxide and keep it in a sort of reservoir. No amount of the gas escapes from it, the researchers say. Nothing escapes - unless you want it to. We believe that this is a turning point in capturing the harmful gas before it reaches the atmosphere." The gas is captured using a new family of materials developed by the researchers and called "Zeolitic Imidazolate Frameworks" or ZIFs (Zeolitic Imidazolate Frameworks).
"Zeolites" are natural and synthetic alumino-silicate minerals that excel in ion exchange properties. They are used to soften water and purify organic substances. have an open crystalline structure and serve as molecular honeycombs. The newly developed materials are porous and chemically stable structures, with a large surface area and it is possible to heat them to high temperatures without their decomposition and to boil them in water or organic solvents for about a whole week while maintaining their chemical stability. The two chemists in his laboratory, one a postdoctoral fellow and the other qualified, synthesized 25 different such structures and successfully demonstrated about three of which showed extremely high selectivity in capturing carbon dioxide: ZIF-68, ZIF-69, ZIF-70. "This selectivity of these structures for carbon dioxide is unmatched by any other known material," says Yagi - a member of the "California Institute for Nanosystems" at UCLA.
"My two synthetics were so successful in making the new structures that when he reported the results I had to ask them to slow down." Within these structures, gas cans can be stored. "Caps" that function as the chemical equivalent of a revolving door allow a certain type of particles - in this case, carbon dioxide - to pass through them into the interior of the reservoir while larger particles or those with other structures are blocked. "We are able to scan and select the one type of mule that we are interested in capturing," says the lead researcher. "The beauty of this chemistry lies in the fact that we have the freedom to choose what type of door we need and to control the components that pass through it." "Capturing carbon dioxide creates "cleaner" energy," says the researcher. "Coating the factory chimneys with these new materials will be able to capture the carbon dioxide inside the pores before it is released into the open air." In the three new zeolites, the scientists emptied the pores in the first step to create a free structure. Then they injected gas jets into the materials - carbon dioxide and carbon monoxide, for example, and another jet of carbon dioxide and nitrogen - and were able to show that only the carbon dioxide remained trapped in the material. The scientists are now looking at similar zeolites for a variety of other applications. In addition to being a greenhouse gas, carbon dioxide causes the death of coral reefs and other marine life, severe and irreversible damage for hundreds of years, the lead researcher adds.
Today, the processes for reducing carbon dioxide emissions from factories also consist of partly toxic substances and require about 20 to 30 percent of the factory's energy. In contrast, the new materials can remove carbon dioxide from other gases that are emitted and are able to store an amount that is five times the amount that other porous materials, the most advanced in this field, are able to store. "Each liter of this material can store 83 liters of carbon dioxide," says the researcher. At the most basic level, the development of these materials also addresses two serious challenges in zeolite science. Zeolites are porous and stable minerals composed of aluminum, silicon and oxygen atoms and which are used in the oil refining industry, in cleaning agents and other products. The research group was able to replace the aluminum and silicon atoms with metal ions such as zinc and cobalt, and the oxygen atoms bridging them with a compound called imidazolate to create the new materials, which can be constructed according to the function or shape required by the scientists.
The two chemists responsible for synthesizing the materials even managed to automate the entire process. Instead of mixing the responders at each step separately and receiving, at best, a few responses each day, they managed to build a procedure of 200 separate responses in less than one hour. The pair of chemists ran 9,600 microreactions of this type and from them managed to discover 25 completely new structures. "We continue to create new buildings like this every other day," says the head of the group. "From these reactions, crystals are obtained that look beautiful and shine just like diamonds."
In the early 90s, Yagi invented another type of new materials called "Metal-Organic Frameworks, MOFs" which were described as "crystalline sponges" and are also used for clean energy. Similar to zeolites, these materials also have pores with nanometer dimensions and are able to store gases inside them that are otherwise difficult to transport from one place to another. Yaji's laboratory has already produced several hundreds of such materials with a variety of properties and structures.

8 תגובות
It doesn't solve anything anyway they will have to release the CO2 into the atmosphere the best solution especially for an area like ours with a lot of sun is to build thermo-solar power plants it is better than a photovoltaic power plant because even for years the efficiency of electricity production does not decrease and then we will not be dependent in oil and we will stop funding our enemies and we will not pollute the atmosphere in short solar energy is the solution
It is also interesting what substances are emitted in the process of creating the miraculous molecule 😉
Cool…
If so, these materials could be used as detectors...
תיקון
Third line from the end should be HYBRID VEHICLES
giving
Photosynthesis is a complete process for absorbing carbon and releasing oxygen into the atmosphere
A technology that uses photosynthesis and solar energy would be preferable
See link
https://www.hayadan.org.il/designer-enzymes-2103083/
Today 99 percent of the machines in the world are CO2 producers
In other words, we humans change the atmosphere and lose ourselves to know
Instead of being in balance with natural resources, we rob and destroy them
Estimated results:
1. Alia in Midbor
2. Extreme weather phenomena.
3. Increase in sea level
5. Wars
6. More poverty
Possible solutions:
1. The countries of the world must give priority in research, development and production of green machines (products that remove materials such as water if at all..)
For electricity generation, water treatment, and vehicles.
Anyone who pollutes (air, water, soil) must be severely dealt with.
2. The use of green machines or a green process (HYBRID VEHICLES) should be encouraged
3. We must gradually stop producing machines that produce CO2 and fine the users in the future.
Maybe the solutions I gave are the answer to reducing CO2 /
Why what's new? Breaking the bond between carbon and oxygen will, as I understand it, consume a lot of energy and there's simply no need for it. I believe that after the gas is contained, it will be gradually released deep in the sea or in large forests that can deal with the natural confinement of the carbon.
It's a good idea but not great
I would expect that after capturing the CO2 they would release the O2 back into the atmosphere.
I didn't understand one thing - what do we do with the gas after that?
Basically it sounds too good to be true