Weizmann Institute of Science scientists have developed a new and simple method for distinguishing between right-handed and left-handed molecules. Possible application: reducing side effects of drugs, pesticides and fertilizers
More than 150 years ago, after discovering that biological systems prefer molecules with a certain direction - left or right - Louis Pasteur proposed to change the direction of molecules by exposing them to a magnetic field. But another great scientist of the time, Lord Kelvin, believed that there is no connection between magnetism and directionality of molecules, and for the first time coined the scientific term for the phenomenon of directionality: "chirality". In the study thatRecently published in the scientific journal Science Scientists from the Weizmann Institute of Science and the Hebrew University of Jerusalem decided the long-standing debate between the two giants of science - in the end, it was Pasteur who was right. These findings gave rise to a new method for separating molecules according to their chirality, a task of enormous importance in the pharmaceutical and chemical industry.
Chiral molecules are a pair of molecules whose structure is the same, but the arrangement of atoms in their space is different. In other words, the molecules are a mirror image of one another, similar to the difference between a right hand and a left hand (the origin of the word "chiral" is from the Greek word meaning "hand"). Although they look the same, molecules with opposite chirality may play completely different roles. For example, a molecule with a certain chirality can function as a drug, but the opposite can cause unwanted side effects. Since during a chemical reaction both right-handed and left-handed molecules are spontaneously formed, when drugs are produced, the molecules must be sorted according to their chirality.
The existing methods for sorting molecules according to chirality are complex and expensive. They rely on technological equipment that is adapted each time to each material. Prof. Ron Naaman from the department of chemical and biological physics of the institute, and Prof. Yossi Palatial from the Hebrew University, together with their colleagues, invented a general, simple and cheap method for sorting chiral molecules of any common substance.
The scientists took advantage of the fact that chirality affects an electron property called "spin", which is characterized by two states - "spin up" and "spin down" - similar to the spinning of a spinning top clockwise or counterclockwise. When chiral molecules come into contact with a surface, they become polarized, following the movement of electrons from one side of the molecule to the other. While in motion, the electrons behave as if they are affected by a magnetic field: the electrons with "spin up" are crowded on one side of the molecule, and those with "spin down" are crowded on the other side. The movement to one side or the other depends on the chirality of the molecule, that is, whether it is right- or left-handed. This phenomenon, whose existence was previously discovered by members of Prof. Naaman's group, is called the CISS effect - an acronym for chirality-induced spin selectivity. It means that chiral molecules can be used as "filters" of spins.
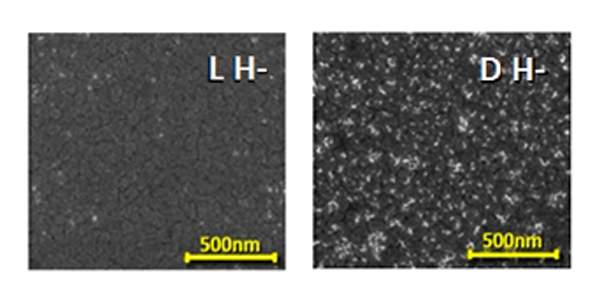
In the current study, the scientists proposed harnessing the CISS effect in order to sort materials according to chirality. They introduced a magnetic surface - where the spins of all the electrons point in the same direction - into a solution containing left-handed and right-handed molecules. When the molecules approached the magnetic surface, they became polarized, and the spins of the electrons in each pole arranged according to the chirality of the molecule. Some were the same as the spins of the electrons on the magnetic surface, and some were opposite, but only the molecules with the electrons with the opposite spins stuck to the magnetic surface; Conversely, those with identical spins were rejected. Thus, the magnetic surface made it possible to sort the molecules according to their chirality, and thus it became clear that Pasteur was right when he believed that there is a connection between chirality and the physical forces acting on molecules, such as those acting as a result of exposure to a magnetic substance.
This method makes it possible to purify drugs according to chirality, thus leaving only the molecules that promote healing - and taking out the harmful ones. In addition, this method can be used to produce more effective chemicals, such as fertilizer and insecticides. In these chemicals, only molecules with a certain chirality perform the desired activity, while those with the opposite chirality are useless and only cause environmental pollution. Sorting by chirality will help produce more effective chemicals, lower their cost, and at the same time also reduce environmental pollution.
To add subtitles in Hebrew, click on the CC button at the bottom of the player
Prof. Naaman's group included Dr. Koel Banerjee-Ghosh, Dr. Francesco Tessinari and Dr. Eyal Kapua from the Department of Chemical and Biological Physics. Prof. Peltiel's group included Dr. Oren Ben Dor, Dr. Shira Yokhelis and Dr. Amir Kapua. Dr. Si-Hon Young and Prof. Stuart Parkin from IBM's research department in San Jose, California also participated in the study; Dr. Sumyajit Sarkar and Prof. Lior Kronik from the Department of Materials and Surfaces of the Weizmann Institute of Science; and Prof. Lech Tomas Baczewski from the Institute of Physics of the Polish Academy of Sciences.
| The production costs of a material that is 98% pure (that is, only 2% of its molecules are in the unwanted orientation) are currently 50 times higher than the production costs of a material that is 90% pure. |

2 תגובות
Now, a bit mean and pointless joke, but really necessary.
For connoisseur readers, I apologize in advance:
Well: the research proves that Calvin was really an absolute zero.
Ha ha ha ……
Indeed a fascinating study like no other and an interesting and instructive article.
Now the following reservation is issued:
Since, as far as is known, about 99% of the organic molecules in nature are chiral, having one polarization (L polarization (left) - in amino acids, and D polarization (right) - in sugar molecules), then in the light reported in the article, it is obvious that the reason for the preference The chirality in the organic molecules in nature is the magnetic field of KDA.
Amazing. How come they didn't think of it before?