Chemists have developed an innovative method for synthesizing chiral molecules that can be used as drugs. The method has already been adopted by researchers in the field of drug development
[Translation by Dr. Nachmani Moshe]
Chemists from the TSRI Research Institute have developed an innovative method for creating chiral drug molecules. "Our process provides a completely new route to the creation of one of the most important structures in the field of chiral molecules, chiral beta-centers, and should accelerate the development of chiral drugs," lead researcher Jin-Quan Yu, a professor in the Department of Chemistry at the TSRI Institute, following the paper published in written - Now the prestigious Science.
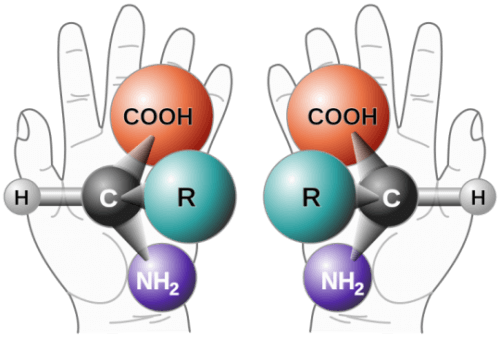
A chiral molecule is not physically symmetrical; It has a "mirror image" that looks slightly different - like a glove on the right hand is a little different from a glove on the left hand. Usually, only one of these formations has the required biological properties - the other may even cause unwanted side effects. Therefore, most modern drugs today contain only one chiral configuration. The ability to do this is not so simple; There are only a relatively small number of useful reactions that lead to a single chiral configuration without giving rise to an equal mixture of the two configurations. As part of the current research, the team faced the problem of adding chiral asymmetry to common organic structures at the ends of which there is a carbon atom bound to two hydrogen atoms through single bonds - in the language of chemists 'beta methylene'.
The ability to selectively convert only one of these two hydrogens in a new group of atoms (functional group) will make the structure asymmetric and allow the acceptance of beta-chiral centers in a wide variety of chiral drugs. However, chemists still do not have a simple method to do this - the common method requires the use of an additional step of creating a double bond between two adjacent carbon atoms. In recent years, the team of researchers has assisted in the pioneering development of a number of strategies that enable the targeting of a palladium atom - whose properties make it an excellent catalyst for breaking bonds - to the exact site in the organic molecule, with the aim of breaking a selected carbon-hydrogen bond. In this case, the researchers developed a chiral catalyst capable of directing a palladium atom to selectively break a bond between the carbon and only one of the two hydrogens attached to it, creating an asymmetric center.
In their research, the chemists were able to remove the hydrogen and convert it into a variety of aryl groups - ring structures commonly found in drug molecules. The catalyst structure includes two ring groups that bind to palladium. The new method works well in two broad and inexpensive groups of starting materials - aliphatic amides and carboxylic acids, while obtaining good utilizations and very high ratios of one chiral configuration compared to the other. In principle, this method can be extended further. "We are currently investigating the possibility of extending our method to other starting materials such as alkyl amines and alkalis," says the lead researcher. "In addition, we are also investigating possibilities that the hydrogen conversion will not only be in aryl groups, but in a much wider variety of organic groups that include nitrogen or oxygen atoms in their content," says the researcher. As part of a commercial agreement, the pharmaceutical company Bristol Myers-Squibb is already using the innovative method in order to create chiral gamma-amino acids required for the synthesis of drugs.