By completing a complex molecular synthesis of macroring compounds linked to a unique identifiable DNA strand, chemists have succeeded in creating a collection of macrorings similar in structure to those that exist in nature, molecules that can be used in the development of new drugs
[Translation by Dr. Nachmani Moshe]
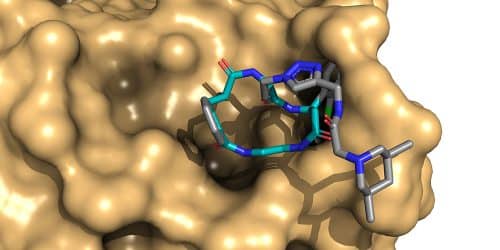
Materials from nature make up the most effective medicines that humanity uses, among them macro-rings with large systems and rich in carbon atoms. The size and complexity of these systems make it difficult to reproduce them in the laboratory. By completing a complex molecular synthesis of macroring compounds linked to a unique identifiable DNA strand, chemists have succeeded in creating a collection of macrorings similar to those that exist in nature, molecules that can be used in the development of new drugs. The research findings have long been published in the scientific journal Angewandte Chemie.
Evolution in nature has led to an incredible variety of small molecular structures that disrupt biological systems, and therefore can be used as drugs. Although only a few dozens of approved drugs are based on macrocyclic structures, they all come from nature or are their close descendants. In order to find promising new compounds in the field of drug research, large collections of substances with diverse structures are required, that is, rich collections of molecules. Chemists in the field of medicinal chemistry have failed to imitate nature's approach to creating macrocyclic molecules with biological activity - and their challenging syntheses hinder the creation of large libraries, which are essential for finding new drugs.
Researchers at the University of Basel's Department of Chemistry have now succeeded in completing a total synthesis of more than one million macrorings with a variety of building blocks common in biologically active macrorings found in nature. The efficient synthesis is based on the principle of "splitting and connecting": before each synthetic step, the entire collection undergoes splitting; And in the next step, each fragmented collection reacts with one of a variety of known building blocks, with each of the new molecules tagged with an identifiable DNA segment. Before the next synthesis step, all segments are joined together again. This process leads to obtaining complete combinations of all the various elements. Each combination is connected to a defined genetic barcode (DNA). Thanks to this efficient approach, all 1.4 million new materials that make up this vast collection can be scanned in a single experiment. Next-generation DNA sequencing of the selected collections could then enable the intelligent identification of macrorings that bind efficiently and selectively to target proteins.
Most drugs based on small molecules are hydrophobic (water-repellent) molecules with a low molecular weight (less than 500 daltons). Therefore, these drugs tend to penetrate through cell membranes and be exposed to most of the proteins associated with the development of diseases. Macrorings work against this trend because they are often extremely large (more than 800 daltons) by medicinal chemist standards, and still penetrate cell membranes tolerably. Researchers hypothesize that this special feature of natural macrorings is derived from their ability to adjust their spatial structure depending on the medium. Thus, in the mostly aqueous environment of the blood circulation and the interior of the cell, the macrorings will place their more hydrophilic groups on the outside in order to remain dissolved in the blood fluid and cellular fluid. Once the hydrophobic cell membrane is exposed to a structural change of the macrocyclic molecule, the membrane allows it to react with the hydrophobic parts of the molecule and penetrate through the membrane which is itself hydrophobic.
In light of their unique properties, macrocyclic molecules are conspicuously underrepresented in the field of medicinal chemistry. This situation is mainly caused by the synthetic challenge of creating a large collection of macrorings for their screening. With the help of a DNA strand used as a code, the research group overcame this setback by developing an efficient multi-step synthesis consisting of seven steps, a synthesis that enables the preparation of a collection of macrocyclic molecules that resemble natural substances, when all are in the same solution. "Thanks to a large and diverse collection of macrorings available to us for screening, it is possible to begin a more in-depth data study of the properties of these unusual molecules," says the lead researcher. "This research could in the future reveal new applications, goals or principles in the field of medicine."
More of the topic in Hayadan:
