Several types of cancer in advanced stages are now successfully treated using artificial immune cells, which are stronger and longer-lived than any immune cell found naturally in the body
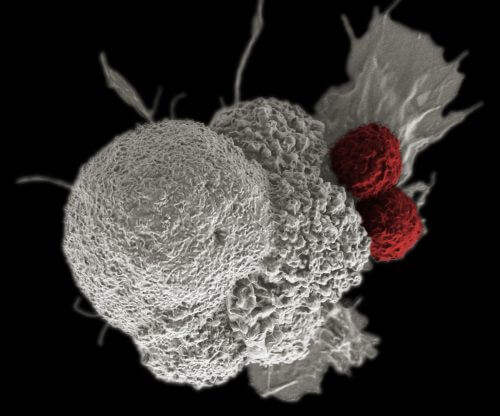
- Artificial immune cells, called CAR T, have been found to be impressively effective in the treatment of leukemia and lymphoma.
- These cells increase and strengthen the body's ability to fight malignant cells.
- But they can cause unwanted side effects, and in some cases even cause death. The researchers now hope to develop new CAR T cells that will be suitable for the treatment of other types of cancer and will cause fewer side effects.
Immunologists who specialize in cancerous tumors have known for several decades that the immune system may be an important ally in the fight against cancer. However, most early attempts to recruit her ability have been disappointing. It turns out that the researchers in the past did not do enough to stimulate key components of the immune system, which function in the system like majors in the army, called T cells. And an immune system going into battle against cancer without increasing the ability of T-cells to recognize and attack cancer cells is likened to an army going into battle equipped with paper airplanes and slingshots.
The first hints that T-cells need a significant boost to be able to fight cancer were discovered during the 80s. The researchers tried to strengthen the immune system's responses by extracting T-cells from the patients' bodies, allowing them to multiply in the laboratory and then injecting the many cells back into the patients' blood. This method helped some patients but not for long. The cells that were returned to the body gradually lost their vitality and ceased to function not long after being put into the body.
Different groups of researchers have tried to tackle the problem in different ways. One strategy, which we and our colleagues have adopted, has recently shown exciting signs of success in clinical trials. In the 90s, while trying to discover new treatments for HIV, two of us (John and Levin) developed an improved technique capable of significantly boosting T-cells taken from patients. The method increased the amount of cells and also made them more powerful and more active than anything that was achieved with the methods prevalent at the time. Then, about XNUMX years ago, we developed a new method of genetically modifying T-cells so that the cells were able to effectively home in and attack certain malignant cells that are characteristic of white blood cell cancers, such as leukemia and lymphoma.
In recent years, these artificial immune cells, known as "T-cells with a chimeric antigen receptor" have been tested (CAR T), in dozens of studies that all together involved almost 1,000 patients who suffered from leukemia or lymphoma in advanced stages. Half or more of these patients, depending on each disease, live longer than expected and it seems that the cancer has disappeared in hundreds of them.
There is a growing consensus among researchers that treatment using CAR T cells, either alone or in combination with other treatment methods, will provide a long-term cure for certain types of blood cancer. In the future, the researchers need to make sure that this treatment is also effective against other types of cancer, and find a better way to regulate the side effects, which in some cases can be fatal. But the success achieved so far, which includes facing a series of difficult challenges over the course of almost 20 years, is certainly encouraging.
Upgraded T-cells
When we set out on the path that eventually led us to CAR T cells, our first task was to find a way to increase the killing power of T cells taken from patients. It was not an easy task at all. In order for T-cells to start working, they must receive signals from another group of cells that participate in the immune system called Dendritic cells. Only after T-cells receive the appropriate signals are they able to realize their full potential. When this happens, they divide many times, produce a huge number of identical new cells (all of which aim to hit the same target) and release from them substances called Cytokines, which add and increase the strength of the body's immune response. A few days later the T cells return to their basic level of function and allow the immune system and the entire body to return to normal function levels.
In the mid-90s, while working on HIV, John and Levine decided to improve this natural process by stimulating T-cells in the laboratory. Our plan was to extract T-cells from a patient's body, activate them, encourage them to multiply to numbers many times greater than they can reproduce naturally in the body and inject them back into that person. They hoped that this would greatly enhance the patient's immune system's ability to fight HIV and other infections that attack a person with AIDS.
But first we had to find an effective way to activate T-cells. Theoretically, it would have been possible to expose them to dendritic cells, which we also isolated from each patient, but dendritic cells differ greatly from person to person in their number and quality, and especially between HIV carriers or cancer patients. To circumvent the problem, we decided to develop artificial substitutes for dendritic cells. After various attempts, we decided to use tiny magnetic balls, and coated them with a coat of two proteins capable of imitating the stimulating activity of dendritic cells, and even improving it.
We then collected T-cells from patients' blood and stimulated them into action using our multipurpose beads. At the end of the process, which lasted between five and ten days, each of our patients' T-cells multiplied and produced 100 additional cells. Our pellet-based method is now one of the primary tools available to researchers to grow activated T-cells, and is widely used in research and clinical trials.
Remodel the T-cell
As the body prepares for an immune response against cancer, it faces two main challenges. One, the origin of the malignant cells in the cells of the body itself. Since the immune system has evolved in such a way that it does not recognize the body's own tissues and does not attack them, it has difficulty distinguishing between cancer cells and normal cells. The second challenge is that many cancer cells are equipped with diverse means of suppressing the immune response. They have means of camouflage that hide them from the cells of the immune system, and they are also able to interfere and disrupt an effective immune response.
T-cells are equipped with a means that prevents them from attacking cells of healthy tissue. A T-cell recognizes a cancer cell by recognizing two molecules found on its outer surface. Identifying these two molecules is necessary to identify the cell as cancerous. Only if they are there, the T-cell attacks the cell. One of them is a large molecular structure called MHC consisting of several proteins. The structure includes a protein component used asantigen: a molecule that the dendritic cells "present" to T-cells and activate them. The second necessary molecule, called a co-stimulatory ligand, provides the signal that tells the T-cells to attack. If one of the two molecules, the antigen-MHC unit or the ligand, is not present on the suspect cell's membrane, the T cell will simply ignore it. If so, a cancer cell has two means of deceiving immune cells: it can stop producing MHC molecules on the surface of the cell membrane, or present a different form of co-stimulatory ligand on its surface that signals T-cells to refrain from activity.
But what will happen if T-cells undergo a genetic change that will allow researchers to take the place of dendritic cells, and choose the target antigen for them: for example, an antigen that is abundant on the surface of cancer cells and is not necessarily presented by MHC molecules? And what would happen if these T-cells didn't have to go through the two-step process to start attacking cancer cells? The development of the technology of CAR T cells made available to researchers for the first time a means that makes it easier for them to try this approach in practice.
Unlike normal T-cells, CAR T-cells attack cancer cells immediately after they recognize their target.
The solution was to match T-cells with genes that would lead to the creation of a synthetic molecule (CAR), capable of doing two things at the same time: recognize the chosen antigen (in the hands of the researchers) and activate a T-cell even in the absence of the usual activation signals. We were able to accomplish this goal by combining protein components that are actually antibodies (which usually attack bacteria and viruses) with additional proteins that are known to stimulate T-cells. More precisely, we designed the antibody-like part of the CAR, which sticks out from the cell membrane, so that it can bind to the selected cancer antigen. On top of that, we assembled the rest of the CAR conjugate, which penetrates through the entire cell membrane, in such a way that it will generate the appropriate signals and activate the cell as soon as it recognizes the antigen unique to cancer.
The idea of attacking cancer-specific antigens to fight malignant tumors is not new, of course. In the 90s, doctors began treating patients with so-called antibodies Monoclonal antibodies, which locates unique proteins found mainly on the cell membranes of different types of tumors. But antibodies only stay in the body for a few weeks. In contrast, when engineered into T-cells, they remain active throughout the life of the T-cell, sometimes for years.
The next challenge was to get the T-cells to produce the desired antibody-activating molecule. We decided to take advantage of the known tendency of HIV viruses to infect T-cells. We removed the genes that make HIV deadly and inserted in their place genes that contain the information needed to build our hybrid molecule, an antibody-activator. Then we allowed the harmless viruses to infect the T cells we removed from our patients. The genetically modified viruses transferred the genes into the T cells; From this stage the cells continued on, creating CAR conjugates and incorporating them into the structure of their cell membranes. (No need for MHC or co-stimulatory ligand.) Moreover, this new T-cell is engineered so that it can hunt down exactly the antigen, or combination of antigens, that the researchers have chosen for it.
In the mid-90s and early 2,000s, we learned, in collaboration with others, to transform T-cells taken from HIV-positive patients into CAR T-cells and tested their activity in human clinical trials. We continue to improve our method and expect to develop more advanced HIV treatments in a few years.
CAR T cells have also begun to be tested in the treatment of cancer patients, by several research groups. We aimed to connect technologies - to use what we learned about activating T-cells using pellets, together with the CAR method, to design and re-direct harmless T-cells and HIV viruses that would act as a kind of friendly Trojan horse that would penetrate the T-cells and insert into them the designated CAR payload.
We soon discovered how powerful these CAR T cells are.
Examining the new planning
We now had a fairly high firepower at our disposal, and we also felt confident that our objective was well established. The perfect signal that would allow our T-cells to home in on the target would have to be, of course, an antigen found only on cancer cells, but such antigens are very rare. Since the origin of all cancer cells is normal body cells, healthy cells and cancer cells usually present the same antigens on their cell membranes. It goes without saying that CAR T cells directed towards the common antigens will destroy healthy tissues along with the tumor tissue.
However, there are some important exceptions. Certain types of leukemia and lymphoma, for example, develop from a group of white blood cells called B-cells. People can live without B-cells, which are the antibody producers in our bodies, provided they receive occasional infusions of prepared antibodies. B-cells, and like them the cancer cells that develop from them, carry a protein called CD19. We and other researchers in the field believed that CD19 might be a suitable target for CAR T cell therapy because it is not found in any other healthy tissue.
First we tested the idea on mice. Later, at the beginning of 2010, we started conducting clinical trials with CAR T cells engineered to target CD19. Three of the first patients were adult patients who were in an advanced stage of chronic lymphocytic leukemia (CLL), and did not respond to other treatments.
The first patient was William Ludwig, a retired jailer who had learned that he was ill ten years earlier, and now he had a huge amount of leukemia cells in his body, about two and a half kilograms, spread throughout his body. In August 2010 he received half a billion of his own cells containing artificial CAR T cells. Ten days later, his fever rose, his blood pressure dropped and he suffered from breathing difficulties, severe side effects that led to his hospitalization in the intensive care unit. We later learned that Ludwig's symptoms were caused by his immune system going into extreme overdrive in response to the large amount of cytokines found in his blood, a response known as Cytokine release syndrome, and it could be fatal if you don't manage to curb it.
Fortunately, Ludwig recovered and a month later the doctors did not find any sign of the presence of cancerous B-cells in his body. This result was unusual and unexpected to such an extent that the doctors repeated the biopsy to confirm it, and indeed received the same result. Then we treated two other patients, and they also had equally surprising reactions. More than six years later, Ludwig and one of the other two patients are still alive and free of leukemia. Further tests showed that the CAR T cells proliferated in the bloodstream and in the bone marrow, the tissue that produces blood cells. Each CAR T cell injected into the three patients' bodies, or its daughter cells, was responsible for killing 93,000-1,000 cancer cells. When CAR T cells were isolated from the patients' blood samples months after treatment, they retained the ability to kill leukemia cells that carried the CD19 molecules under laboratory conditions. In fact, these shields behaved as a long-term "living drug", which continues to circulate in the body and capture every reappearance of a malignant cell.
expanding the repertoire
Despite the significant initial results we achieved, our budget ran out and we could not try our experimental treatment on more patients. Committees of federal research agencies that examined our work determined that the treatment was too dangerous and did not warrant additional funding. Nevertheless, we submitted two articles describing the results of the first three patients, and the articles were quickly accepted and published together in August 2011 in the New England Journal of Medicine and Science Translational Medicine. The publication resulted in an extensive review in the media, as well as great interest from the start-up groups and biotechnological companies who approached the University of Pennsylvania, where we worked, and were interested in the possibility of obtaining a license for the technology.
In the end, one of our grant requests was answered positively and thus we were able to conduct another clinical trial that began in 2012, this time in children. Later we decided to establish a partnership of the University of Pennsylvania with a company Novartis To finance the continued development and submission of the results to the FDA to obtain approval for commercial use of the method. The partnership set off a mad race of many medical centers around the world forming new biotech companies dedicated to producing new versions of CAR T cells. Our latest results in children show survival rates of 62% after one year, compared to survival rates of less than 10% after one year, due to Standard treatments.
Over the past few years, many groups, including the Salon-Kettering Cancer Center, the Seattle Children's Hospital, the Fred Hutchinson Cancer Research Center, have reported joining the company Juno Therapeutics, the National Cancer Institute, which is a member ofKite pharma, and others, about surprising reactions of leukemia and lymphoma patients in advanced disease stages. In our center, we have treated 300 patients with CAR T cells that are treating B-cell malignancies. The response rates vary depending on the disease: about half of our patients who have chronic lymphocytic leukemia in an advanced stage show a clear clinical improvement (based on a decrease in the number of leukemic cells in their body, along with other factors), while about 90% of children with acute lymphoblastic leukemia (ALL) showed a complete response: the absence of signs of the presence of cancer cells one month after treatment.
We do not know why CAR T cell therapy does not work in all patients with CD19-bearing malignancies. In some cases, there is a withdrawal probably because the CAR T cells did not multiply in the patient's body, or because new leukemic cells appeared that do not produce CD19 and are therefore not affected by the treatment. Even so, the extent of the response to the treatment of these cancers is unprecedented. In 2017, it is expected that two companies will ask the FDA to approve the use of CAR T cells for the treatment of cancer: Novartis, for the treatment of acute lymphoblastic leukemia in children and later for lymphoma, and the Kite company for the treatment of a certain type of lymphoma.
Many challenges still face us. The research community is still busy developing ways to alleviate and possibly prevent the worst side effects. Even if deaths among the patients are rare, some patients who suffered from acute lymphoblastic leukemia died due to treatment-related problems, most likely due to their general health which was particularly fragile, and perhaps also due to differences in the structure of the CAR T cells used in the various research institutes.
We are now in the phase "Model T” of developing CAR T cells. Our first priority is to expand their availability to patients with B-cell cancer and other tumors, and several recent scientific and technological innovations will be tested in clinical trials in the coming years. To treat cancers that are not associated with B-cell malignancy, researchers probably need to find combinations of certain antigens that are more common on cancer cells than on cells of healthy tissues, and focus on them. One of us, for example (Posey), is trying to develop a treatment based on the immune system for breast cancer and pancreatic cancer. These and other cancers, which are diseases of solid tumors, are good at hiding and suppressing the natural immune system, more so than lymphoma and leukemia, which are more vulnerable to treatment because their cells circulate in the blood system. To locate the cells of solid tumors, Posey is designing a CAR T cell that will target two targets instead of one: one is a certain sugar molecule that is only found on the membranes of cancer cells and allows them to multiply faster than healthy cells; The second is a protein molecule found on both healthy and cancerous cells. Theoretically, the combination of the sugar molecule with the protein is expected to be found with high frequency only on cancer cells, which should limit the ability of this particular CAR T cell to harm healthy tissues.
Progress in this field rarely occurs in a straight line. Disappointments, wrong assumptions and delays are inevitable. But we have no doubt that the success already achieved in the treatment of leukemia and lymphoma justifies the continued research and development of additional CAR T cells.
Disclosure: Like many other cancer researchers, the authors have commercial relationships with several for-profit companies. Avery D. Posey owns intellectual property and has licensed its use to Vertis and Tmunity Therapeutics, which develops cancer treatments. Carl H. John and Bruce L. Levin receive royalties and lab funding from the Vertis company, in exchange for a license allowing them to use intellectual property and an agreement with the University of Pennsylvania. Vertis and the University of Pennsylvania have applied for drug patents based on some of the research work summarized in this article. John and Levine are co-founders and shareholders of Tmunity Therapeutics and they also receive consulting fees and advise several companies involved in cell therapy and cancer research. These connections are managed in accordance with the policies of the University of Pennsylvania and are subject to its supervision.

2 תגובות
Amazing video