A liquid-viscous environment helps the folding of proteins and their proper functioning
Many of the proteins in our body - which are made from a chain of amino acids - fold into an orderly three-dimensional structure that gives them stability and allows them to function. Failures in folding cause them to be unable to perform their function. Usually, such faults are caused by a genetic defect (mutation) or environments (solutions) in our body that make it difficult for the proteins to fold. Thus, due to the malfunctions in folding, neurological diseases such as Alzheimer's develop and other diseases such as sickle cell anemia and cystic fibrosis.
Prof. Daniel Hariz from the Faculty of Mathematics and Natural Sciences at the Hebrew University of Jerusalem is a chemist who, together with his team, studies solutions that simulate the environment of living cells, and their effect on the molecules in them. These solutions include water and large molecules such as proteins, DNA (made up of nucleic acids), lipids (fats) and polysaccharides, as well as small molecules such as sugars, salts and additional metabolites (metabolic products).
"We take solutions, add large molecules to them, mainly proteins, and check what will happen to them, to their stability and function," says Prof. Hariz. "In the past, biochemists studied molecules such as proteins in dilute solutions, which mainly included water. We build on their research, but study rich and dense solutions, so that they better simulate the environment of the body's cells and help understand how proteins exist in it. When proteins are constrained by density, they are generally more stable and function better. The study of proteins in a dense environment is essential not only for understanding their folding and function and for curing and preventing diseases, but also from a technological point of view. Through it you can learn about the stabilization of large molecules, such as proteins, polysaccharides and lipids, for industrial needs. For example, food is made up of such molecules, and their stabilization can contribute to its preservation and extending its shelf life."
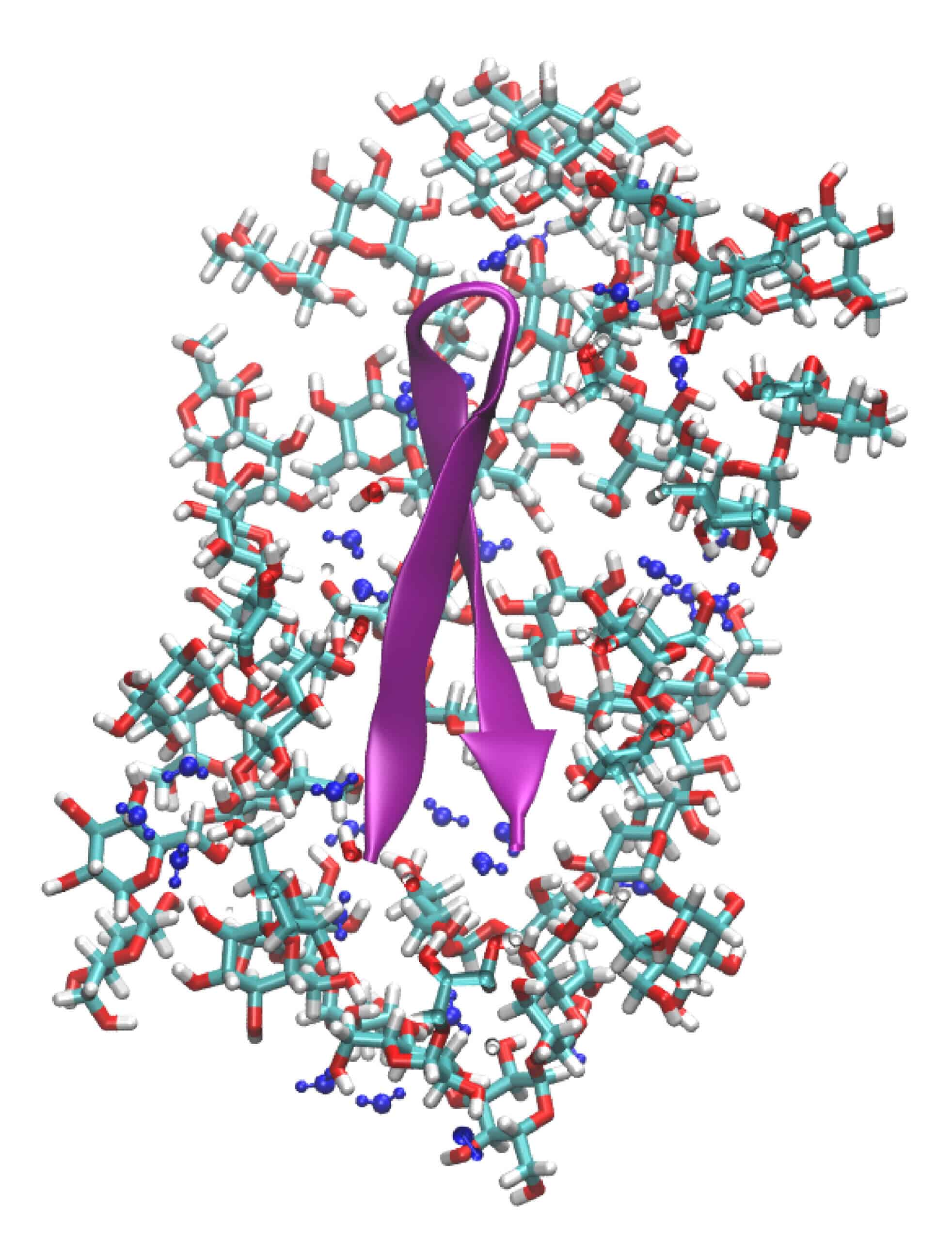
In their experiments, Prof. Hariz and his team dry the rich and dense solutions they prepare, so that they become a kind of viscous liquid - the so-called "glassy state". It is known that many animals manage to cope with stress situations by producing a glassy biological environment in their body cells. This glassy environment contains a mixture of large and small molecules. For example, when water bears (tardigrades) suffer from dry conditions, their bodies produce a high concentration of molecules (such as sugars). Thus, when the liquid inside evaporates, a glassy cell environment (in which the molecules are embedded) is created. In this way they can survive for many years.
In a study that won a grant from the National Science Foundation, the researchers, including Prof. Hariz and team members Gil Olgenblum and Liel Sapir, sought to understand how the glass environment allows for the preservation of the function of large biological molecules - such as proteins, DNA and lipids - and thus contributes to the preservation of body cells and the survival of Life such as the water bears. For this purpose, they put together a dense solution that contains mainly proteins and mono- and di-sugars. Then dry it so that it becomes vitreous, and focus on the study of the MET16 protein found in it (which is derived from a protein whose function is to bind DNA).
They chose it due to its simple structure (reminiscent of a hairpin). Thus, they could relatively easily follow the changes in its structure in the glassy state, using spectroscopic methods (light frequencies) and computer simulations. That is, they were able to see how the vitreous-cellular environment affects it. This is how they discovered that the folding of the protein did not change. That is, it remains the same as its fold in a thin liquid environment, stable and normal.
However, the researchers discovered that the unfolded state of the protein (before it folds) becomes compact in the glass environment, and even takes on properties of the desired folded state. This, in contrast to what happens in the liquid environment, where the protein's unfolded state is messy and bulky. This difference is due to the high concentration of sugars and the almost complete absence of water in the glass environment.
"This study reveals to us that the glass environment helps proteins to fold better," says Prof. Hariz. "In fact, even when they are unfolded - they do not move away from the folded state. From this it can be understood that this environment helps the proper folding of proteins and thus it may prevent diseases and pathologies that result from its disruption, and also contributes to the stabilization of large molecules. In the future, we hope that these findings will enable the development of medicines and advanced methods of conservation."
Life itself:

Prof. Daniel Hariz, 51 years old, lives in Jerusalem and is fond of calligraphy, calligraphy (the art of decorative writing), and ink drawing.
More of the topic in Hayadan:
