In the study published today in the scientific journal Cell, Weizmann Institute of Science scientists demonstrated an innovative therapeutic approach that performs "targeted defeat" of unwanted intestinal bacteria using a creative and precise weapon - viruses that attack bacteria
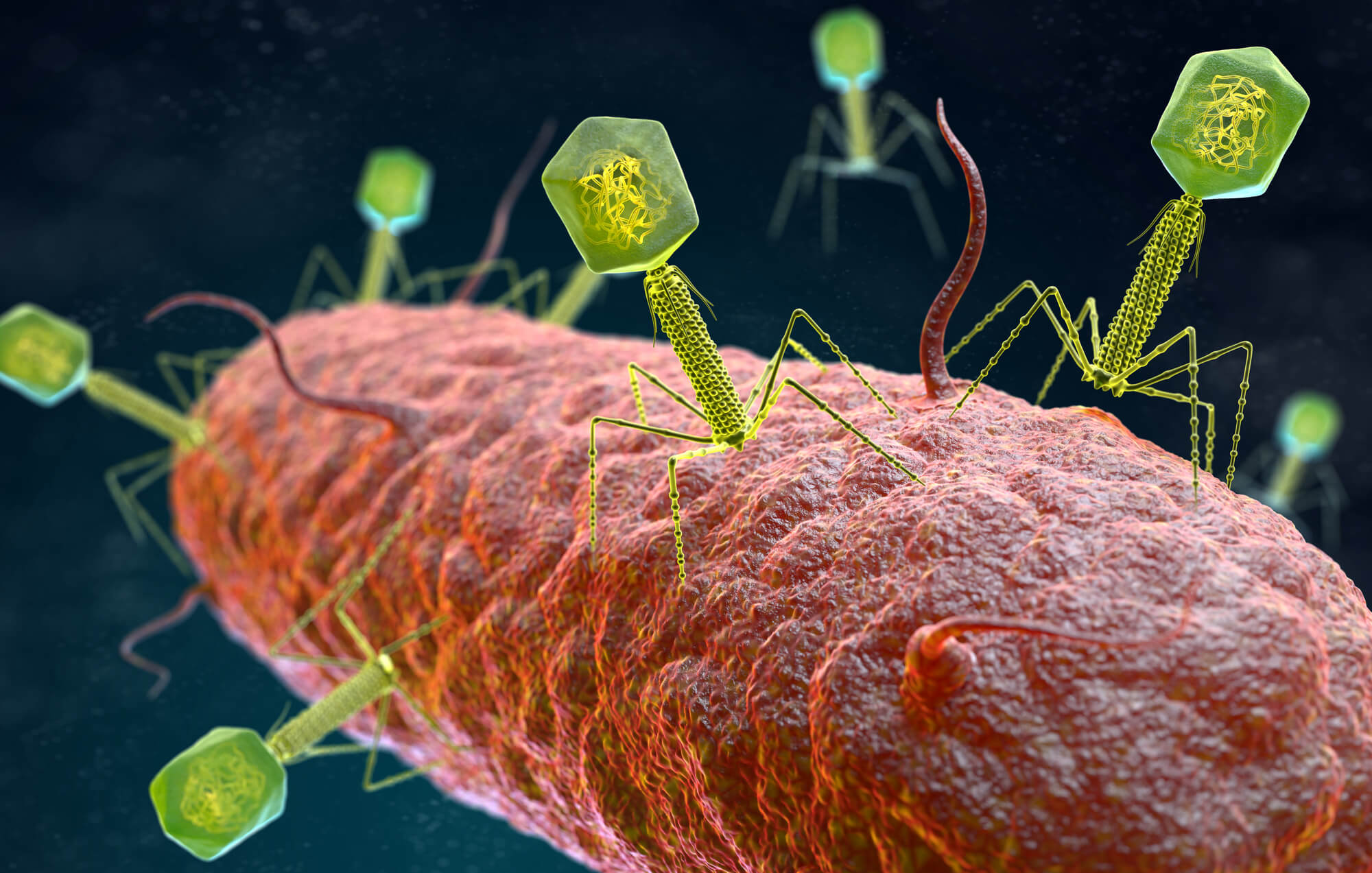
The millions of bacteria residing in our intestines are essential for our health, but less friendly bacteria may infiltrate their ranks and contribute to the development of inflammatory bowel diseases and many other diseases. Today, it is not possible to eliminate the harmful bacteria without harming the entire bacterial population, known as the microbiome, since antibiotic drugs do not know how to distinguish between good and bad bacteria. Moreover, the use of these drugs involves side effects and contributes to the rise of antibiotic-resistant bacterial strains. in research published today in the scientific journal Cell, Weizmann Institute of Science scientists demonstrated an innovative therapeutic approach that performs "targeted defeat" of unwanted intestinal bacteria using a creative and precise weapon - viruses that attack bacteria.
Viruses that attack bacteria, also known as bacteriophages or phages for short, are found everywhere there are bacteria, including the human intestine, and are actually one of the most common organisms on Earth. Already in the early 20s of the last century, when they were first discovered, attempts were made to harness the phages to combat disease-causing bacteria, but these studies were soon abandoned with the discovery of antibiotics.
"There are thousands of types of phages in nature, and each of them specializes in attacking a different family of bacteria," explains Prof. Eran Alinev from the department of systemic immunology of the institute who headed the research team. "This fact paves the way for the development of 'surgical' treatments that target only unwanted bacteria. To the best of our knowledge, the therapeutic approach we developed is the first that promises to eliminate disease-causing intestinal bacteria without harming the entire bacterial population, i.e. the microbiome."
The research conducted in collaboration with the laboratory of Prof. Rotem whistles from the department of molecular genetics at the institute, led by Dr. Sara Federici, Dr. Raphael Valdes Maas and Dr. Denis Koiatkowski from the Alinev Laboratory, as well as Dr. Sharon Cardo-Russo and other researchers fromBiomix" - a start-up company that is in the stage of clinical trials for the development of phage-focused medical treatments, based on the research of Weizmann Institute of Science scientists, and with an exclusive license from the company "ידע", the institute's intellectual property commercialization arm.
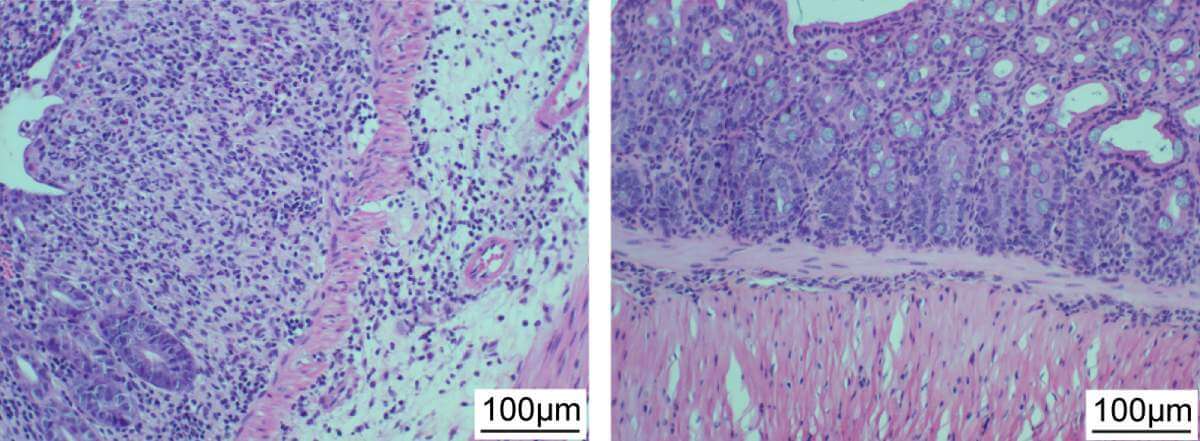
To find the appropriate phages, the scientists first had to map the strains of bacteria involved in inflammatory bowel disease. The team compared the composition of the intestinal bacteria of healthy volunteers and their composition in patients with the two most common inflammatory bowel diseases - ulcerative colitis and Crohn's disease; To neutralize effects of place of residence and origin, participants from France, Germany, Israel and the United States were included in the study. A careful analysis of the findings using a computer model led the scientists to a series of possible suspects - bacterial strains that did not characterize healthy participants, but appeared in large quantities in patients, especially during periods of exacerbation of inflammatory bowel disease. Some strains of Klebsiella pneumoniae (Klebsiella pneumoniae) were identified as the main suspects. These suspicions were confirmed when the bacteria were implanted in mice with intestinal infections: they caused the inflammatory condition to worsen and the damage to the intestinal tissue to increase.
Armed with these findings, the scientists could move on to the next step – scanning thousands of phages to find the candidates that would best fight the harmful bacteria. About 40 phages have been identified as particularly active against the strains of bacteria involved in intestinal infections, but that is not the end of the job: bacteria have millions of years of experience in dealing with phages and are able to neutralize them very efficiently. To make sure that the phages would have the upper hand, the scientists used the latest scientific discoveries about the defense systems of bacteria and tried to find the best combination of phages - one that could slip through the defense lines of the bacteria. They eventually formulated a cocktail of five phages selected based on their genetic profile and structural features. Working together, the fabulous five were able to overcome various strains of Klebsiella pneumoniae, including strains resistant to antibiotics.
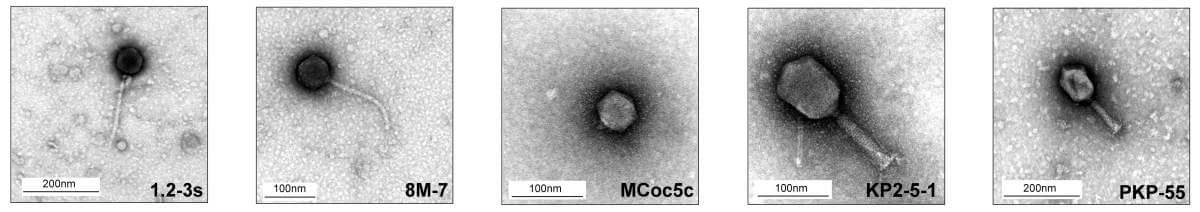
The phage cocktail was found to be effective against Klebsiella pneumoniae strains extracted from patients both in vitro and in mice with intestinal infections; In these mice, the cocktail led to a reduction in signs of inflammation, tissue damage and mortality. Also, a laboratory system simulating the conditions prevailing in the human intestine revealed that the phages are stable and may survive inside the body when given in combination with antacids. These encouraging findings led to the first phase of a clinical trial in which 18 healthy volunteers took part. In the experiment it was discovered that the phages do not cause significant side effects, and that they survive in the intestine for a long time and even multiply - and all this without leading to unwanted changes in the microbiome.
As long as the phage cocktail is found to be safe and effective even in clinical studies with many participants, it may form the basis for developing treatments not only for inflammatory bowel diseases, but also for other syndromes affected by the intestinal bacteria, including obesity, diabetes, degenerative brain diseases and possibly even cancer. "Our vision is to develop personalized treatments for a wide range of health problems," says Prof. Alinev. "Identify harmful bacterial strains in each patient and put together their personal phage cocktail."
Also participating in the study were Dr. Meli Dori-Bakhsh, Dr. Hagit Shapira, Claudia Morsi, Amanda Cubes, Dr. Gayatra Mohapatra, Dr. Lara Kern, Dr. Dunping Zeng, Dr. Samuel Philip Nobbs and Dr. Yotam Suez from Prof. Alinev's lab; Dr. Nisa Kolin from the German Cancer Research Center (DKFZ) in Heidelberg, Germany; Eyal Weinstock, Dr. Yulia Matyohin, Dr. Yael Zilberberg, Dr. Ido Viner, Efrat Kabara, Noa Ben-Yashi, Dana Inbar, Dr. Chava Ben David, Dr. Julian Nissenbaum, Dr. Nega Koblesman , Dr. Eidit Kario, Tal Cohen, Yael Friedman Gefen, Dr. Lior Salzbuch, Dr. Ariel Cohen, Dr. Ornia Rapo, Dr. Einbar Gahli Shesh, Dr. Miriam Golambo, Dr. Vared Lev , Dr. Naomi Zack, Dr. Sailja Potgunta and Dr. Merv Basan from the "Biomix" company; Dr. Koji Atrashi, Dr. Munahiro Furuichi and Prof. Kenya Honda from Keio University School of Medicine in Tokyo; Dr. Akihiko Oka, Dr. Bo Liu and Prof. R. Balfour Sartor of the University of North Carolina; Dr. Maureen Pibelman and Prof. Nitzan Maharshak from the Sackler Faculty of Medicine of Tel Aviv University; Dr. Noa Stettner and Prof. Alon Hermlin from the Department of Veterinary Resources of the Weizmann Institute of Science; Prof. Harry Sokol of Sorbonne University; Prof. Wolfgang Lieb, Dr. Corinna Bang and Prof. Andre Franke from the Christian Albrecht University in Kiel, Germany, and Prof. Christoph Schramm from the Hamburg-Eppendorf University Medical Center.
More of the topic in Hayadan:
