Chemists from the University of UCLA in the USA and a university in South Korea have reported on the "ultimate degree of porosity of a nanomaterial", achieving world records both for the degree of porosity and for the storage capacity of carbon dioxide in an important type of material family called "organic-metallic frameworks"
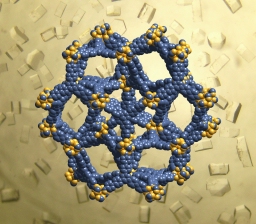
Materials from the family of organic-metallic skeletons (the entry in Wikipedia), which are sometimes described as "crystalline sponges", contain tiny pores at the nanometer level that are able to store gases within them that are usually difficult to store and release. The porosity property is a decisive factor for storing large amounts of gases in small volumes and is essential for capturing carbon dioxide.
The findings of the new research could lead to the development of cleaner energy and the possibility of capturing carbon dioxide emissions to prevent them from reaching the atmosphere and thus continue to contribute to global warming, to raising the sea level and increasing the acidity of the oceans. The study was published in the prestigious scientific journal Science.
"In this study we report the ultimate porosity rate of a nanomaterial; We believe that this rate represents the upper limit, or very close to it, of porosity in materials," said the paper's lead author Omar Yaghi, a professor of chemistry and biochemistry at UCLA.
"Porosity makes it possible to do a lot with a little," notes the researcher. "Instead of using only the outer surface area of a particle, we make tiny holes in it and thereby significantly increase its total surface area."
In this study, the scientists report on two materials that not only break the world record for porosity, but also do so by a large margin. The two materials are MOF-200 and MOF-210, prepared at Seoul University in South Korea.
"Not only have we provided a major advance in the study of these materials," says the lead researcher, "but these two materials are special in that they approach the practical limit that can be achieved in this family of materials. Maybe we can design better buildings, but it won't be easy."
These materials, developed by the researcher Yaghi himself in the early 1999s, are like scaffoldings made up of rods tied together, and which thereby create nozzles that are exactly the right size to capture particles of carbon dioxide. The components of these materials can be changed at will and the laboratory of these researchers has already prepared several hundred of these materials, with diverse structures and properties. Since XNUMX, these materials have the highest porosity of any material and can be produced from cheap starting materials, such as zinc oxide (a typical ingredient in sunscreen) and the material terephthalate, from which plastic bottles are made.
The scientist and his research team discovered the key to preparing highly porous structures, which were reported in the scientific journal Nature back in 2004. Since then, chemists have begun a scientific race to develop materials with increasingly large surface areas. Now, the researchers have prepared a highly porous material capable of storing twice the amount of gas that existed in 2004. for the trapped gas," explains the researcher. "It's really magical - forty tons of the material is equal to the surface area of the entire state of California."
"And this is just the beginning of the use of these materials," he adds. "Because now we can use them as a template for building new materials and developing innovative properties. The requirements for preparing an effective material for capturing carbon dioxide are high capacity and selectivity. We reported how high selectivity for carbon dioxide can be achieved; We now show how high capacity can be achieved. The industrial applications for these materials have already begun to be developed. Many companies are engaged in the development of materials from this family."
The new materials (MOF-200/210) capture the largest amount ever achieved also for the gases hydrogen and methane.
In February of this year, the researcher, and a team of other scientists from UCLA University, published in the scientific journal Science how they managed to create a synthetic "gene" capable of capturing carbon dioxide emissions. Based on the findings of the new research, it is now possible to develop the synthetic gene with the new materials and thereby achieve a much larger surface area. "This family of materials is unique and unprecedented in its versatility in number, diversity and composition options."
The key was in researcher Hiro's discovery of how to completely remove the solvent particles from the nozzles and thus allow them to be used to capture the gas particles," explains the lead researcher.

6 תגובות
I am interested in the shape of the crystalline sponges, are they indeed in the shape of a Star of David as photographed
Haim,
If you had thought a little instead of jumping, you would have seen that Sharan surfed the site on Monday, the upgrade took place on Tuesday night and you surf to it on Thursday.
And besides, your logic is completely unclear. If you yourself claim that "the plants in the world barely manage to recycle the CO2", then how exactly does its capture make the situation worse?
Once again we see irresponsible scientists in action.
The amount of oxygen on Earth is small and limited. There are about 200 grams of oxygen per square meter. Every day we burn 2.7 billion kg of fuel, most of which is carbon. The plants in the world barely manage to recycle the CO2.
Go out and think when the oxygen will run out on Earth if they start storing the carbon dioxide.
So, the irresponsible so-called scientists add insult to injury with an irresponsible crazy thesis.
Leran M: Your computer is broken. Browsing the site is fine. As for what to do with the CO2, start using green energy. sun, wind Sea, waterfalls and even nuclear energy.
On Tuesday night, the site will be upgraded on several fronts at the same time. Let's hope that one of them will be the one that improves the speed. We moved to a server with 4 cores, and now we check the software to see if there is something that has stopped loading pages slowly.
DA, what do you do with the carbon after you capture it?
Abi, you must invest money in normal servers.
Browsing the site is unbearable. Sometimes it takes ten or more seconds for a page to load. In a world where normal websites load in less than a tenth of a second (and heavy ones take a second) this is an eternity.
There is no doubt that it hurts the popularity of the site.