Hydrogen may be one of the most important fuels in the future. It would be most preferable if it could be obtained from water decomposition instead of oil. However, electrolysis of water is a process that requires a lot of energy and makes it both expensive and ineffective if the electricity required to operate it comes from burning fossil fuels. Photolysis, breaking down water using light, is a very promising replacement process
A team of Australian and American researchers have now developed a catalyst that effectively catalyzes the essential half of this reaction, the photooxidation of water. As reported in the journal Angewandte Chemie, the core of the new catalyst is a conjugate containing the element manganese, which mimics that found in organisms that carry out photosynthesis.
Electrolysis is the reverse process that occurs in the battery: that is, electrical energy is converted into chemical energy. The electrolysis of water consists of two half-reactions: at the cathode, protons are recycled to hydrogen, while at the anode the oxidation of water produces oxygen. The goal of the researchers was to use sunlight to activate this energetic process. In order for this to work, it is necessary to combine the modern solar cells that use the sun's energy with efficient photocatalysts to oxidize the water and reduce the protons to obtain hydrogen gas.
The highest hurdle that had to be crossed to obtain photocatalytic decomposition of water, until now, was finding an efficient catalyst that oxidizes water. In fact, the best known catalyst, which oxidizes water most efficiently in response to light radiation, is an enzyme in the photosynthesis system of living organisms that contains manganese.
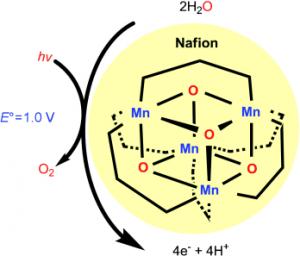
The researchers used exactly this enzyme as a model for their research - the catalyst is an oxo-manganese conjugate with a cubic core that includes four manganese atoms and four oxygen atoms covered by secondary phosphorus compounds. The active catalytic form is created when light energy causes the release of one of these compounds from the conjugated structure.
However, the manganese conjugate is not soluble in water. The researchers overcame this problem by coating one of the electrodes with a thin film of the Nafion material. When placed within the aqueous channels of this membrane, the catalyst is stable and makes contact with the water particles. Irradiation with visible light, when a current of 1.2 volts is applied, leads to an efficient electro-oxidation of the water.
This anodic half-cell could easily connect to a catalytic cathodic half-cell that produces hydrogen. The combination will result in a photoelectrochemical cell that can produce pure hydrogen and oxygen from water and sunlight.

9 תגובות
The problem of the night may be partially solved, in areas developed in terms of infrastructure, by flowing electricity from lit places to unlit places while utilizing the existing networks. It is possible to flow electricity from east to west and vice versa.
During the day, all businesses are open, not only manufacturing plants, and the air conditioners also work hard, so a solution for the daytime is also a quite practical solution.
The main problem is not so much the night hours, but rather the evening hours when there is no more sun but there is not a lot of activity (high-tech, shopping malls).
Today, different methods are being developed for storing solar energy during the daylight hours by utilizing it during the dark hours, it seems to me that there was an article or discussion on this subject in science not long ago.
In a few years when the technology of utilizing solar energy will be advanced enough it will be used to produce most of the required energy.
I do not despise cold fusion, but to point to it as "the right way" when it is not at all known whether it can be done and how it looks to me is hasty, to say the least.
The idea here describes exactly….energy storage. The fact that (without the help of satellites) sunlight can only be used for half of the day does not mean that it is not possible to produce enough energy for a whole day.
Cold fusion is the right direction to create energy
Just proves that evolution is one of the most powerful tools of technological efficiency today
To my people:
Dependence on sunlight for energy production is not the best solution for the simple reason - the sun is not available throughout the day and therefore energy storage is necessary - which drastically lowers the efficiency of such a system.
In my opinion the preferred solution is "private sun" as an energy source 🙂 AKA cold fusion
Such a system will produce a lot of energy and there is no need to store fuel / energy
Unfortunately, since the last fiasco on the subject, the investment in research in this direction has decreased, but I hope that the bitter memory will be erased and they will return to invest in this direction.
Well..you see the right direction..and there is no such thing that does not have a solution.
Hugin: In the name of the original "Ami".
Oh well... we'll live and see.
Is the mechanism of the release of water into oxygen clear and known as can be understood from this article? Is this process completely understandable to us? If so, when will we find out?
And the other question is, is it possible to make of this a kind of leading Perpetum in which the system will also provide itself with the necessary electric current and at the same time harvest the hydrogen.
In fact, the day we know how to turn water into energy using sunlight, all our energy problems will disappear altogether. But then, of course, completely different problems will come.
Greetings friends,
Ami Bachar