In order to map the population of viruses in the intestines, the researchers used a surprising "catalogue": a bacterial immune system that was discovered about five years ago, and is thoroughly studied in the laboratory of Dr. Rotem Sorek at the Weizmann Institute

Despite their bad name, bacteria may be beneficial creatures. The number of "good" bacteria in the human gut is 10 times greater than the number of cells in the entire human body, and the evidence is growing that they play an extremely important role in our health - they produce vitamins for us, aid in digestion, and also "train" the immune system, thus reducing the incidence of allergies and diseases Autoimmunity.
In many environments outside the intestine, bacteria are attacked by viruses called "phages", which are able to kill large amounts of bacteria in a short time. Are intestinal bacteria also exposed to viruses? And if so, who are these viruses, and which bacteria do they harm? These questions - which until now have not received much attention - were the focus of the latest research by Dr. Rotem Sorek from the Department of Molecular Genetics, which was published in the scientific journal Genome Research.
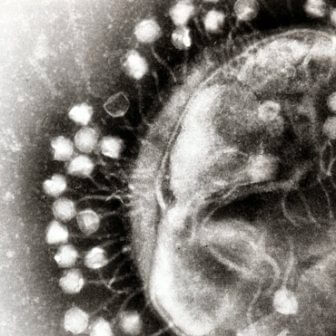
A scanning electron microscope image of phages attacking a bacterium. Photo: Dr. Graham Bird, Wikimedia Commons
In order to map the population of viruses in the intestines, the researchers used a surprising "catalogue": a bacterial immune system that was discovered about five years ago, and is thoroughly studied in Dr. Sorek's laboratory. This system, called CRISPR, is designed to provide protection to bacteria against viruses. When a virus penetrates a bacterium, the system "steals" a small piece of the genetic material from it, and stores it within defined immune sequences in the bacterium's genome. These sequences help the bacterium identify and destroy the virus the next time it invades the cell, similar to the principle of how antibodies work in the human body. These immune sequences were used by the researchers as a sort of "historical record" of the viruses that attacked the intestinal bacteria.
The team of scientists, which included (then) postdoctoral researcher Dr. Adi Stern, and undergraduate student Eran Mick, used a large database, which contained DNA sequences of intestinal bacteria collected from samples of 124 European people. They developed a computational method that makes it possible to identify the immune sequences within the DNA of the bacteria, and found more than 50,000 such sequences. Later, the scientists used the immune sequences to identify hundreds of viruses that attack different intestinal bacteria, which were previously unknown.
These findings - which constitute the largest pool of phages from the gut found by researchers to date - shed some light on the connections between intestinal bacteria, viruses, and the humans who harbor them. Thus, for example, the researchers discovered that large groups of people share the same viruses, and that about 80% of the viruses were found in the intestines of more than one person. A comparison of samples taken from Americans and Japanese revealed that they also share the same types of viruses. Considering the diversity and great variety of viruses found in other systems, this is a surprising finding, and the scientists speculate that the reason for this is the closed environment in the intestine. Another discovery is that the viruses are sometimes carried in their entirety within the DNA of the bacteria. "The phages often donate genes to the bacteria that make them resistant to antibiotics, and 'in exchange' for that the bacteria store their DNA and pass it from person to person," explains Dr. Sorek. "This is a successful evolutionary 'deal' for both parties."
The database of phages now in the hands of the researchers, which allows them to identify which phage infects each bacterium, opens the door to studies that will examine their effect on human health. For example, if a certain bacteria helps us acquire resistance to an allergy, it is possible to check which virus attacks it, thus causing the outbreak of the allergy. Dr. Sorek: "The next step will be to help the bacterium defend itself against the virus by using the bacterium vaccine against it. In this way, the immune system of the bacteria may help, indirectly, the human immune system."
The good, the bad and the bacteria
Listeria bacteria
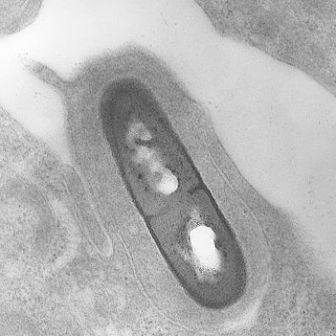
In biology, as in archaeology, moving from layer to layer may completely change the picture of reality. Comparing the RNA of two species of the Listeria bacterium - one of which causes food poisoning and disease in humans and the other is harmless, instead of the usual comparison of the DNA sequence, revealed a whole set of differences that were hidden from view until now. In addition, and "by the way", this study, which was recently published in the journal Molecular Systems Biology, discovered a basic biological mechanism that was not known until now, which controls the activation of genes in the Listeria bacterium.
The team of scientists, which included Dr. Rotem Shurk and his group members, Omri Wurtzel, Dr. Iris Koronker-Hazan and Sharit Flor-Adelheit, used advanced sequencing methods to map the total RNA of the two species of bacteria, focusing specifically on in those sequences that do not code for the creation of proteins. Despite the genetic similarity between the two species, the scientists discovered a group of about 80 RNA sequences that exist in the disease-causing bacteria, but not in the "peace-seeking" bacteria. Later, the researchers plan to check if these sequences do indeed contribute to the bacterium's violence.
In addition, the scientists discovered pairs of RNA sequences, organized so that the expression of each inhibits the other. According to the researchers, this is a unique mechanism that controls the activation and silencing of genes, which may be common in bacteria.

One response
For the most part, the phages destroy the bacteria, which they infect. That is, they cause lysis. In this way, even if the bacteria create a kind of databases about the viruses that penetrated them, it is very doubtful if they will survive to deal with a phage the next time.
It is more likely that infected bacteria transfer the same viral DNA segments to neighboring bacteria in the flora by conjugation, for example, thus "vaccinating" them.