Chemists from the Netherlands have discovered that an enzyme with a unique structure that has never been observed, does exist in reality: two ring structures interlocked in a form known as catenanes. The research findings were published in the scientific journal Chemical Communications.
Chemists from the Netherlands have discovered that an enzyme with a unique structure that has never been observed, does exist in reality: two ring structures interlocked in a form known as catenanes. The research findings were published in the scientific journal Chemical Communications.
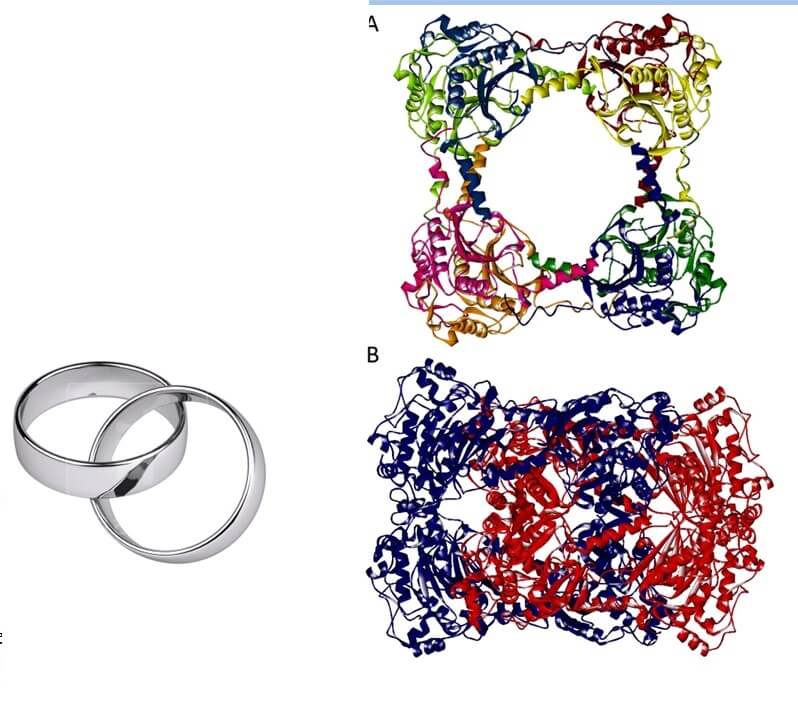
Microbiologist Mike Jetten from the Dutch Radboud University Nijmegen is an expert in finding new bacteria and archaea that perform unusual chemical reactions. In 2011, he discovered a primitive bacterium in volcanic mud originating from volcanoes in Italy that emits hydrogen sulfide (H2S) and carbon dioxide (CO2) as a result of a reaction with carbon disulfide (CS2) and in the presence of the enzyme carbon disulfide hydrolase (CS2hydrolase). Structural analysis revealed that the enzyme consists of two forms: structures of a single ring and structures of two rings entwined with each other, in a general structure known as hexadecamericcatenanes.
The interlocking rings are very special - their locked protein structure, based on weak non-covalent interactions, has never been seen in biology. This is the reason why the researchers initially had to examine whether these structures are indeed connected to each other or if they are two free rings that intertwine randomly from time to time. A chemist from the research group was responsible for checking whether this integration is indeed permanent and real, or random and occasional.
"By dissolving the enzyme we were able to check the ratio between the amount of single rings and the amount of double rings at low concentrations. If the double rings were indeed an occasional and random event, we would not expect to find them in solutions with low concentrations since the chance of the rings joining together randomly is very small. We found that the ratio was constant in all the different concentrations, a finding that proves that the combined rings are indeed a permanent and not random structure. We performed this test using three different methods: chromatography based on the size of the molecules, multi-angle laser light scattering and mass spectrometry." The three separate methods strengthen the findings obtained by each of the methods and now the researchers know for sure: the combined rings do exist.
"We have an unusual array of proteins," says the lead researcher. In the previous decades, many chemists meticulously checked whether small molecules in the form of catananes do exist, but we initiated research on extremely rare biological catananes. In fact, there is not a single article on this topic, so there are many fundamental questions that still await resolution. Now, we are trying to understand why Mother Nature chose this unique structure. It is possible that this structure developed during biological evolution or that it has certain advantages compared to other enzyme structures in terms of its rate of catalysis and its stability. However, it may just be a chemical coincidence. We are interested in understanding this through an even more careful examination of the structure of the double rings."
The news about the study
