Intervening before the onset of symptoms may be the key to stopping the main cause of delirium or slowing it down
By Gary Stix
In the book One hundred years of loneliness The magical-realist masterpiece of the Colombian author Gabriel Garcia Marquez, the author takes the reader to the mythical jungle village of Condo, where, in one of the most quoted scenes, the inhabitants suffer from a disease that causes them to lose their memory completely. The disease erases "the name and meaning of things and finally the identity of people." The symptoms persist until a traveling gypsy arrives with a "soft-colored" drink that restores their lives.
In the city of Medellin in Colombia, as a kind of realistic equivalent of Macondo, perhaps several hundred residents of the city and its surroundings will get an opportunity to help in the search for an actual version of the gypsy potion. In Medellin and the surrounding coffee growing areas live the largest group of people with hereditary Alzheimer's disease. Women and men from 25 extended families, numbering 5,000 family members, develop early-onset Alzheimer's disease, usually before the age of 50, if they carry a defective version of a particular gene.
Early-onset Alzheimer's, which is inherited as a dominant genetic trait from only one parent, accounts for less than 27 percent of the 2006 million Alzheimer's cases recorded worldwide in 65, but the characteristic changes in the brain appear to be the same as those of the more common disease, whose symptoms do not appear until after age XNUMX.
The ability to anticipate the onset of the disease in families from Medellin has attracted the attention of scientists and pharmaceutical companies, who are investigating the possibility of testing drugs by giving them to patients before they show the first signs of the disease.
In recent years, several potential drugs for the treatment of mild to moderate Alzheimer's disease have failed, so the researchers came to the conclusion that the pathology of the disease - the accumulation of damaged proteins and the loss of cells or circuits in the brain - begins long before the impairment of memory is detected. Following this understanding, which was confirmed by new technologies that detect the disease years before the appearance of the first symptom, everyone agreed that the treatment should be started even in the years when the disease progresses without any external signs, when the patient's memory is still completely normal.
Therefore, a significant portion of Alzheimer's research is currently dedicated to stopping the disease before the symptoms appear - not only through drugs, but also through a correct lifestyle, which will be safer and cheaper than 10 to 20 years of drug consumption.
early start
The Colombian Alzheimer's families are the vanguard of prevention research. Francisco Lupera - a neurologist who 28 years ago first encountered these families, who were later discovered to be carriers of the Paisa mutation (paisa is a nickname for the people of the area) - contacted hundreds of family members who had not yet fallen ill to test their willingness to participate in a trial of drugs that would eliminate or stop the accumulation of the protein fragments The toxic, amyloid-beta type peptides, which damage brain cells during the disease. "The knowledge that these families will contribute may shed a lot of light on the treatment of Alzheimer's disease and its prevention in both its early and late onset," says Lopera.
As part of an extensive effort called the "Alzheimer's Prevention Initiative" (API), an experiment is scheduled to begin in 2011 with members of the families carrying the mutation: healthy people around the age of 40 will begin receiving anti-amyloid treatment (a drug or vaccine) that has already been proven safe among Alzheimer's patients. The intention is to send a cyclotron - a small particle accelerator - to the area that will be used by several hospitals in Medellin to prepare radioactive tracers for the imaging tests that will reveal if the drug inhibits the accumulation of amyloids.
The experiment will examine whether providing treatment to the carriers of the defective gene seven years before the average age at which their disease is diagnosed can delay or stop the silent and decisive course of the disease. In addition to examining specific treatments, the Columbia trial planners want to see if tracking Alzheimer's-specific biomarkers will make it possible to find out if the experimental treatment is helpful. (A biological marker is a measurable indicator, such as the concentration of a certain protein, that changes with the progression or regression of the disease.) A reliable group of such markers will allow the drug researchers and treating physicians to assess the success of the treatment fairly quickly, and there will be no need to wait for visible symptoms. The API is planning a similar series of trials in the United States among a group of carriers of two copies of a mutant gene, APOE4. This gene increases the chance of Alzheimer's, but does not predict with certainty that the disease will actually break out.
If the API is successful, it will pave the way for many Alzheimer's prevention trials based on biomarkers. Proving that a drug prevents a disease takes much longer and costs much more money than showing that it cures those who are already sick. "A pharmaceutical company will not invest in a long-term prevention trial of an unproven substance that may turn out to be ineffective," says Maria Carrillo, director of scientific and medical relations at the Alzheimer's Association.
With a set of biomarkers in hand, a pharmaceutical company could test whether a drug changes amyloid levels or another biomarker, just as doctors test cholesterol levels as a measure of a statin's effect in preventing heart disease. "We must promote treatment before symptoms appear. Otherwise, we could lose an entire generation," says Eric M. Reiman, director of the Banner Alzheimer's Institute in Phoenix, who initiated the API with his colleague Pierre N. Tariot.
Clinical trials to test the prevention of a disease pose many difficulties: it is difficult to weigh the inevitable side effects of the drug against the potential benefit to a person who does not yet have symptoms. Furthermore, no one can predict whether a drug that is beneficial for early Alzheimer's disease will also benefit people who do not have the particular mutation that causes the early onset of the disease. But the urgent need to find new treatments - and the lure of a drug that brings in billions of dollars - motivated the development of prevention strategies. In January 2010, an API meeting was held in the USA, and 19 representatives of pharmaceutical and biotechnology companies from the USA and Europe were present. The discussion revolved around the possibility of creating a non-competitive alliance between academia and industry, which consists of cooperation in clinical studies and the free transfer of experimental results.
There are treatments for Alzheimer's, but they do not significantly slow the progression of the disease. A treatment that will make a real change in the disease will be in great demand. Statisticians predict that by the middle of this century the incidence of Alzheimer's in the world will increase fourfold and the number of patients with the disease will reach 107 million. A treatment that delays the onset of the disease, even for five years, will cut the number of deaths from it in half.
inside your head
Until about five years ago, an Alzheimer's prevention trial based on biological markers was accepted as complete fantasy. But thanks to imaging methods and other technologies today it is possible to track biological markers and reveal the nature of the disease process. Since 2004, the "Alzheimer's Disease Neuroimaging Initiative" (ADNI) has been operating in the US, in which pharmaceutical companies, academics and the National Institutes of Health (NIH) collaborate. What began as the development of methods for improved evaluation of the effectiveness of drugs among subjects who already had the disease, soon expanded to examine the events during the period before the actual diagnosis.
One intriguing report of progress in the field came on January 21, 2010: Clifford R. Jack, head of the ADNI group investigating biomarkers that can be monitored with magnetic resonance imaging (MRI), described a possible model of the course of the disease and attached it to biomarkers that appeared to They have the ability to track this pathology. Jack presented his work, which also appeared in a technical journal, in an Alzforum webinar in front of more than 100 participants, including many of the leading researchers in the field. The seminar, one of whose founders is June Kinoshita, a former editor at Scientific American, is a meeting place for the exchange of ideas, a repository of research information and a source for in-depth articles on Alzheimer's research.
In the webinar, Jack noted that the measurements of the biological markers suggest that the process begins years before the onset of the symptoms by which the disease is diagnosed. During this period of time (estimated at about 5 to 20 years), a certain type of amyloid peptide begins to accumulate outside brain cells, and it damages the synapses, which are the meeting points between nerve cells. A radioactive tracer molecule, such as PIB (Pittsburgh imaging compound-B), binds to the amyloid in the subject's brain, and then its location can be discovered in the images using positron emission tomography, PET. With this method, it became clear that the accumulation process slows down before the appearance of distinct symptoms.
Then, still before the diagnosis, a group of proteins called tau, which in its normal state helps provide structural support to nerve cells, was detached from the cell skeletons. The proteins get tangled up, become clumps and wreak havoc inside the cells. The tau nodules are detected by testing a sample of spinal fluid. This test can also detect a decrease in amyloid-beta levels, which occurs when the peptides leave the fluid and form plaques in the brain. When you find a decrease in the levels of amyloid-beta and an increase in tau in the cerebrospinal fluid, this most likely means that the disease process has already begun.
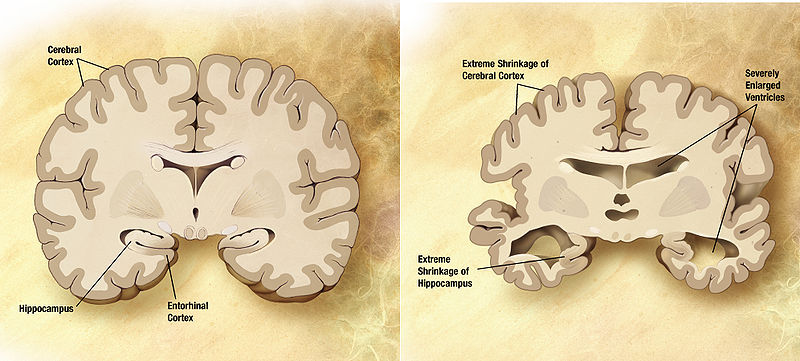
Between one and four years before the diagnosis of the disease, a stage called "mild cognitive impairment" begins. The symptoms that characterize this stage range from episodes of memory loss to poor decision making. Mild cognitive impairment may be due to other reasons, not Alzheimer's. In any case, patients progressing towards Alzheimer's dementia suffer mild cognitive impairment when nerve cells in certain brain areas are damaged or die - and the process accelerates over time. (If memory problems are the main symptom, the patient usually progresses towards Alzheimer's.) At this stage, it is possible to follow up with an imaging method called volumetric MRI, which measures the rate of shrinkage of the brain due to nerve cell death. The chain of events, including the early amyloid accumulation, disrupts the cell's metabolism. Monitoring at this stage is possible using a special PET test - fluorodeoxyglucose-PET (FDG-PET) - which assesses the metabolic state of nerve cells.
Is the patient's condition improving?
The basing of clinical trials to test the prevention of the disease on biological markers poses difficult challenges both to the pharmaceutical companies and to the legislator and is a barrier to the breakthrough of the API and other prevention efforts. In order for a drug for Alzheimer's to be approved for use, it will be necessary to show that the cognitive benefit it brings to the patient (in memory, language or a similar measure) is greater than that of a dummy drug (placebo).
If in a prevention study a biological marker is followed and not symptoms, the researchers have to be sure that the measurements do show the subject's chances of developing insanity. For example, researchers still do not know whether changing amyloid-beta levels will ultimately prevent dementia, despite the large body of evidence showing that amyloid-beta contributes to the development of the disease.
In fact, in an early trial of amyloid treatment, the levels of the peptide decreased in some of the patients, but there was almost no sign of improvement in cognition. "We are concerned that we may have a drug that affects the marker as we expect it to, but does not affect the clinical picture of the patients," says Russell Katz, director of the Neurology Products Unit at the US Food and Drug Administration. "In other words, their disease continues to develop, and there is no improvement in their condition." Katz says that in order to integrate biomarkers into clinical trials, it is better to first show that lowering the level of amyloid or another biomarker is beneficial to people with mild cognitive impairment or who have been diagnosed with Alzheimer's disease, and only then try to use biomarkers to treat people without symptoms. "The best way to reach the goal, in my opinion, is to start with patients who have symptoms, perhaps patients in the very early stages, and then work backwards," says Katz.
But the researchers conducting the prevention trials at Columbia say with confidence that they can already detect slight changes in memory using biomarkers, and Katz can relax. Reiman cites work by his group that suggests another way to assuage lawmakers' concerns. In psychological tests done in the same study, carriers of the gene version showedAPOE4 A slight decrease in the memory rating many years before any cognitive deficit became apparent. Such a level of sensitivity, Reiman says, means that a combination of a cognitive test and measuring a biological marker in a prevention trial may be enough to indicate whether a decrease in amyloid levels does indicate a better chance of not reaching dementia. For now, Katz is still not convinced: "What is the evidence that these patients, despite their poor cognitive condition, will indeed go on and develop Alzheimer's disease?" He says.
Several companies are already investigating the use of biomarkers. Bristol-Myers Squibb takes samples from the cerebrospinal fluid of mildly cognitively impaired patients to try and predict which ones will develop Alzheimer's. Those who are found to have a low level of amyloid-beta and a high level of tau will be suitable to participate in a trial of a drug that blocks the enzyme gamma secretase, which is involved in the production of the amyloid-beta peptide. "If you don't have the biomarker linked to the pathophysiology of Alzheimer's, you are not eligible to be treated in our study," says Vlad Couric, medical director of the Global Clinical Research Department at Bristol-Myers Squibb. The ability to focus only on patients with a high chance of developing Alzheimer's will help to assess whether the drug is indeed beneficial - an assessment that would have been less clear if participants with a low chance of getting the disease had been included in the study. "In the future, drug research may begin even earlier, in the pre-symptomatic phase," adds Couric.
Cognitive workshop
The Colombian Alzheimer's families at the heart of the API were also the inspiration for another innovative prevention approach. Neuroscientist Kenneth S. Cusick, who worked with the Colombian families for almost 20 years and helped identify the pisace mutation, founded a center in 2009 that he calls a "cognitive workshop" in a residential neighborhood in Santa Barbara, California. It was Kusik who organized an important meeting in Medellin to introduce Lopra and the Colombian families to the API.
The Cognitive Workshop - whose official name is the Center for Fitness and Innovative Cognitive Therapies (CFIT) - is a safe haven for both those who experience mild memory problems that sometimes develop into full-blown Alzheimer's and those who fear for no known reason. These people come to the stylish establishment to receive, based on the latest evidence, advice on changes they can make in their lives to ward off the menacing shadow of insanity or better cope with it if it arrives.
Kosik adopted the CFIT idea from another center, Casa Neurociencias, an unassuming outpatient clinic next to the Central Hospital in Medellin, where he spent many hours working alongside Lopra. Alzheimer's patients with the Paisa mutation, often accompanied by dozens of family members, would come from the village on a long bus ride to spend the day in the open space of the clinic, where the medical staff and family members had easy access to each other. "It is interesting that there, in the medical system that is not so developed, personal care and auxiliary services were more available," says Kosik.
Kosik compares the therapeutic atmosphere there to the clinical efficiency of Harvard Medical School, where he co-founded a clinic for memory disorders before moving to UC Santa Barbara in 2004. "I was frustrated that people would come to the clinic and we would tell them, 'Yes, it looks like Alzheimer's,' and then hello and that's it," he says. "We would see them once every six months, but we couldn't do much except document their deterioration."
CFIT combines the informal atmosphere of Casa Neurociencias with healthy lifestyle recommendations, many of which are based on an evolving body of scientific knowledge emerging from new, epidemiological or animal studies, suggesting that various activities may aid cognition. Epidemiologists follow a select group to determine whether exercise, diet, or many other activities may reduce the chance of diseases such as Alzheimer's, although more structured types of studies are needed to draw firm conclusions.
After a physical and psychological assessment, the client (the word "patient" is never used) receives a series of personal recommendations, including, for example, consuming a Mediterranean diet (healthy fats and a high consumption of vegetables and fruits), doing aerobic exercise and playing online mind games. The center hosts some activities that are not yet accepted in places like the Harvard-affiliated Memorial Clinic. In the new reality, where patients want more control over the medical care they receive, Tonya Kayland, a cognitive psychologist, helps people navigate the tangle of medical information on the Internet. She projects a web browser onto the wall of the darkened meeting room, goes through with the client, page by page, clinical trials or new research on turmeric or another dietary supplement that may protect brain cells and explains the weight of evidence regarding one compound versus another.
CFIT also coordinates tests for the presence of the gene variant APOE4. The test, which arouses controversy, is performed after advising the client about the implications of knowing the result: if it is positive, siblings and offspring may also carry the gene and are at high risk. Many medical groups oppose the test because knowing the genetic status does not make it possible to determine unequivocally whether a person will develop Alzheimer's, and because there are no really useful treatments available.
Kosik, who was one of the authors of one of the first articles on the tau protein, denies that he has become a "spa" doctor who promotes questionable ideas. His lab at UC Santa Barbara is still researching the tau protein and other esoteric basic biology. CFIT intends to fill the gap until the API or another initiative can discover drugs or other means of proven efficacy. "The solutions we have here are not the best solutions," says Kosik, "but we don't know when the drug will arrive that will treat the disease the way penicillin treats the infection. I think it's irresponsible to tell people that this will happen in five or ten years, because I don't think we know."
In the coming years, the CFIT approach to prevention will be tested in rigorous, state-sponsored clinical trials that will reveal whether diet and exercise can indeed delay the disease, or whether the epidemiological evidence was just a statistical duck. "One of the main questions about lifestyle," says Reisa Sperling, a professor of neurology at Harvard Medical School, "is whether an intervention affects people whose brains are still normal and people whose brains are already showing Alzheimer's changes differently. Some of the interventions may lower the risk, but if you are already on your way there - if you have the genes and if your head is already full of amyloid - they may not be so successful in slowing the progression of the disease, so it is important to test these ideas with biomarkers to see if they Really helpful.”
In the end, a PET or lumbar puncture may determine whether olives, goat cheese, and half an hour daily on the treadmill will help preserve cognition, or if they are just a flower crow. If these biomarkers prove useful, the biological and behavioral studies may coalesce into the real science of Alzheimer's disease prevention.
___________________________________________________________
key concepts
The incidence of Alzheimer's disease continues to increase as the population ages, but no effective treatments have yet been found.
Medications that have failed may prove to be effective if given at an earlier stage.
New methods for monitoring the disease before symptoms appear will allow drugs to be tested at a stage when they might be more effective.
Alzheimer's in numbers
The expected flood
With the aging of the population in the United States and the world, the number of new Alzheimer's patients is expected to soar because the incidence of the disease increases with age. According to the estimate, today there are more than 39 million people over the age of 65 in the US, and by 2050 the number is expected to reach 89 million, more than double.
The population is aging…
Millions of people aged 65 and over living in the USA.
2050
Today 2010
1900
...and age is the main risk factor for Alzheimer's...
The risk of men and women developing Alzheimer's at a given age in the next ten years.
... and therefore the number of Alzheimer's patients is increasing
The number of people diagnosed with Alzheimer's will increase by about 50% in the next 20 years.
2000: 4.7 million
2010: 5.3 million
2030: 7.9 million
100 years of research
1906: The German psychiatrist Alois Alzheimer describes for the first time (based on an autopsy of the brain) the protein plaques and tangles of nerve cells that characterize the disease.
The next 50 years: Memory loss and other symptoms are considered senility that results from normal aging.
1960-1970: A link between cognitive deterioration and the number of layers and tangles of nerve cells in the brain is proven.
1980-1990: Researchers begin to discover the basic biochemistry of the formation of the layers and tangles.
1990-2000: Genetic causes of the disease are discovered. The first drugs that improve the symptoms go on the market.
2000-2010: Imaging and lumbar puncture allow scientists to track the course of the disease. Some drugs to treat the disease process fail in clinical trials, and many conclude that treatment should be started at an earlier stage.
Medicines today
A little relief, but not enough
Today, the drugs only treat cognitive symptoms and not the disease process that causes them, and they work for a limited time - from a few months to a few years.
Type of drug:
acetylcholinesterase inhibitor (examples: sonepezil, galantamine)
what is she doing?
Blocks the action of the enzyme acetylcholinesterase and thus increases the level of acetylcholine in the brain. Excess acetylcholine improves cognition, mood and behavior, and thus day-to-day functioning improves.
Type of drug:
NMDA receptor antagonist
(one drug: memantine)
what is she doing?
Helps dampen overactivity through a signaling molecule, glutamate, which may lead to nerve cell death. The drug does not interfere with the formation of the cell lesions that promote the disease.
Progress towards prevention
New tools reveal hidden early signs
The process underlying Alzheimer's disease begins years before the onset of the symptoms that allow a diagnosis. Researchers can now follow him with tools that monitor biomarkers associated with Alzheimer's disease, such as brain imaging and lumbar puncture: signs of biological changes (such as increased levels of toxic proteins) that always occur during the course of the disease. Researchers hope that one day, tests of biological markers will make it possible to discover the disease in early stages, and the treatment in these stages will prevent the madness or slow it down.
Amyloid accumulation
5-20 years before the diagnosis of Alzheimer's dementia
At an early stage, pieces of protein called amyloid-beta accumulate in the centers of the brain that create new memories. The accumulation of amyloid, a biological marker detected through the presence of plaques, causes damage to the synapses that connect nerve cells (detail). Amyloid prevents chemical signals (neurotransmitters) from reaching the receptors on the receiving nerve cells. The accumulation can be seen in various forms of brain imaging, such as positron emission tomography (PET), which identifies a radioactive compound - Pittsburgh imaging compound-B (PIB) - which binds specifically to amyloid. Lumbar acupuncture can also be used to monitor the amyloid marker.
Tau accumulation
1-5 years before diagnosis
Before the symptoms justify an Alzheimer's diagnosis, tau proteins inside the nerve cells begin to behave wildly. In a normal state tau helps to maintain the structure of the tiny tubes (microtubules), which is essential for the normal functioning of nerve cells. But now phosphate groups begin to accumulate on tau proteins (pert), and these are detached from the microtubules. The microtubules disintegrate and the tau proteins aggregate and form coiled clumps that disrupt cell function. The process can be discovered in a spinal fluid sample.
Brain shrinkage
1-3 years before diagnosis
As the disease progresses, nerve cells begin to die, and the patients and their families feel impaired in memory and other cognitive functions. Cell death shrinks the brain in areas associated with memory (in the hippocampus) and higher brain functions (cortex), and this can be monitored with a type of magnetic resonance imaging that measures brain volume. The degeneration accelerates and eventually reaches many areas of the brain.
treatment status
Why the treatments are not helpful
Any drug that significantly delays or stops Alzheimer's will be an instant hit, and its sales may exceed those of Prozac or Lipitor. There are no such drugs on the market because researchers are still trying to figure out how to change the mechanism by which the disease causes delirium.
An example of this is the drugs that inhibit the build-up of amyloid plaques: several drug candidates, which are in various experimental stages, are apparently capable of inhibiting the accumulation of amyloid or speeding up its elimination. However, some amyloid drugs tested in clinical trials have already failed. Some researchers wonder if until now we have not given due consideration to the interference with other processes that contribute to the disease. Among the approximately 100 different substances currently in development are several drug candidates that act against tau proteins that damage nerve cells. Some of them are designed to reduce inflammation, speed up mitochondrial function, increase the level of insulin in the brain or protect nerve cells in other ways. The last big failure was Dimebon, a substance that actually did not work against amyloids. It may be necessary to combine several substances to slow or stop Alzheimer's, as is done in the development of drugs against cancer and AIDS.
Medicines in research
what are they doing
Amyloid-beta-producing enzyme inhibitors
Certain enzymes cut large proteins and release amyloid-beta fragments. The inhibitors block these enzymes or change the way they work.
Vaccine components or antibodies that clear plaques of amyloid-beta
The ingredients activate the body to produce antibodies that bind to amyloids and remove them from the brain. Unfortunately, both the ingredients and the antibodies caused side effects of varying degrees of severity in some of the subjects in the clinical trials.
Blockers of amyloid-beta aggregation
Substances that prevent amyloid fragments from clumping together may prevent damage to nerve cells.
Anti-tau agents
Although the number of these agents is smaller than the number of those directed against the amyloid pathway, they work in several ways, such as blocking the production of the toxic form of the tau protein and inhibiting its entanglement into bonds.
Nerve pro-active substances
Several different approaches try to stimulate the brain's production of natural chemicals that improve nerve health. In one of the preparations, a gene is inserted into the brain that starts the production of a protective substance.
Import from Colombia
The neurologist Francisco Lopera built a standard for the treatment of the large group of families affected by a hereditary form of Alzheimer's at a clinic in Medellín, Colombia. This community-centered treatment approach, founded by Lopera and his colleagues, inspired the "Cognitive Workshop" in Santa Barbara, where clients are involved in programs of physical exercise and other activities designed to reduce the risk of dementia.
And more on the subject
The Alzheimer's Project: Momentum in Science. Based on the HBO documentary. John Hoffman and Susan Froemke, with Susan K. Golant. Public Affairs Books, New York, 2009.
The Alzheimer's Solution: How Today's Care Is Failing Millions and How We Can Do Better. Kenneth S. Kosik and Ellen Clegg. Prometheus Books, 2010.

5 תגובות
Read a little more learned things about coconut oil here:
http://scienceblogs.com/insolence/2011/01/dr_oz_finally_unequivocally_embraces_the.php
In general - all these stories of concealment are simply delusional.
In the future, an article by Shermer about conspiracy stories will be published here.
It may sound strange to those who haven't heard of it before, but 2 tablespoons of coconut oil a day per patient stops the spread of the disease. Google "coconut oil and Alzheimer's". Those who take care of an Alzheimer's patient and of course the patient himself has nothing to lose here. The drug companies do not want this information to reach the general public because it is a lot of money that they will lose if they stop buying their drugs. In short, 2 tablespoons of coconut oil per day. It's simple, cheap, available, and most importantly, it helps.
It may sound strange to those who haven't heard of it before, but 2 tablespoons of coconut oil per day for the patient stops the spread of the disease. Google "coconut oil and Alzheimer's". Those who take care of an Alzheimer's patient and of course the patient himself has nothing to lose here. The drug companies do not want this information to reach the general public because it is a lot of money that they will lose if they stop buying their drugs. In short, 2 tablespoons of coconut oil per day. It's simple, cheap, available, and most importantly, it helps.
Tired of this conspiracy mantra.
Adversary: What are you doing about it besides making claims to others?
How much money are they going to make...more decades of people without symptoms receiving drugs.
Not that I'm saying not to use it, but instead of trying to find a solution that will really solve the problem, it seems that the efforts are being taken in the direction of enslaving people to their drugs..