Lego-like fibers formed due to a single mutation in a protein may serve as a scaffold for nanostructures

The proteins in our body are sociable "creatures" - they communicate with each other non-stop. But similar to relationships between people, the relationships between them are also undermined from time to time, and new relationships are formed in their place that may harm our health. For example, just one mutation in hemoglobin - the protein that carries the oxygen in the blood - is enough for the hemoglobin molecules to stick together, similar to Lego blocks, and produce long and stiff fibers. These fibers cause the red blood cells to elongate, which is exactly what happens in sickle cell disease.
For more than 50 years, sickle cell disease was the only example known to science of a mutation causing the formation of Lego-like fibers, but Dr. Emmanuel Levy And the members of his group in the Department of Structural Biology of the Weizmann Institute of Science hypothesized that these fibers should have appeared more frequently during evolution. asRecently reported in the scientific journal Nature, the researchers did show that it is easy to make proteins organize themselves into a Lego-like structure. The new findings may have implications for biomedical research and the study of nanomaterials.
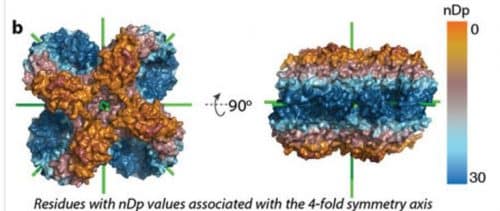
Dr. Levy and his group drew their inspiration for the research from the symmetry that enables the adhesion of hemoglobin. Clusters of symmetrical proteins are built from identical units - even if you turn them over they will continue to look the same, a bit like an Oreo cookie. And since identical units are produced by the same gene, any mutation that occurs in the gene repeats itself many times in the protein cluster - and because of the symmetry, is expressed on both sides. If this mutation causes adhesion, the molecules will continue to stick to each other more and more, producing longer and longer fibers. In this process, the building blocks of the aggregates retain their original shape - in contrast, for example, to amyloid aggregates which change their shape in the process of producing the "plaques" that characterize Alzheimer's disease.
The mutation that causes adhesion leads to a change on the surface of the protein: it replaces a hydrophilic ("water-loving") amino acid with a hydrophobic ("water-hating") amino acid. When the protein molecules move in their aqueous environment, the hydrophobic segments prefer to interact with each other.
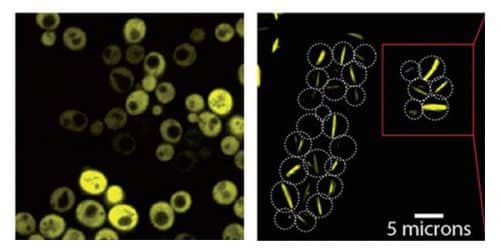
Dr. Levy and his group - Dr. Hector Garcia Seysados and research student Charlie Emporor-Mot along with Dr. Nadav Elad from the Department of Chemical Research Infrastructures - first conducted experiments with a symmetrical protein cluster consisting of eight identical units. "In this way, for every mutation in the gene of the protein, we received 'for free' seven additional mutations in the pool created," explains Dr. Levy. The mutation caused only one change in the protein: replacing a hydrophilic amino acid with a "sticky" and hydrophobic amino acid. They caused such mutations in the genes of 11 symmetrical proteins - between one and three mutations in each of the genes - and caused these genes to be expressed in yeast cells with the addition of a fluorescent protein that allows tracking. In 30 of the 73 versions of the proteins created in the experiment, a phenomenon similar to self-assembly was observed - the creation of Lego-like fibers. About half of these proteins organized into ordered fibers, and the other half stuck to each other in amorphous clumps. When the scientists photographed the yeast cells through the microscope in order to monitor their growth, they saw that clusters of fibers were formed in these cells along the entire surface of the cell, from one end to the other.
But the question arises: if the scientists were able to reproduce with such ease the formation of fibers similar to the fibers formed in sickle cell anemia, why has this phenomenon not been observed until now in biomedical research? There are two possible answers to this: First of all, the institute's scientists discovered that proteins that are naturally symmetrical have many hydrophilic amino acids on the surface, which reduces the risk of self-assembly. In addition, Dr. Levy believes that scientists may have already seen such adhesions in the past, but assumed that these were proteins with a disordered fold - without biological significance. "After we found out how easily such compositions can be formed, maybe now other scientists will start to recognize them in different situations."
Dr. Levy further explains: "The uniqueness of our research is in its simplicity: we did not use complex calculations and did not scan thousands of mutations in order to find the appropriate one. We started with an existing structure and used a simple method to make the fibers self-assemble. If it is possible to induce the formation of fibers in yeast in such a simple way, perhaps these fibers can be used as a skeleton of nanostructures".

One response
Are the amount and types of proteins similar in all living things and also in relation to the DNA that encodes in its code the assembly of the proteins themselves and what about plants