High in the sky, water in the clouds is able to act as a means of coaxing forcing airborne pollutants, such as sulfur dioxide, to become active aqueous constituents (as acid rain).
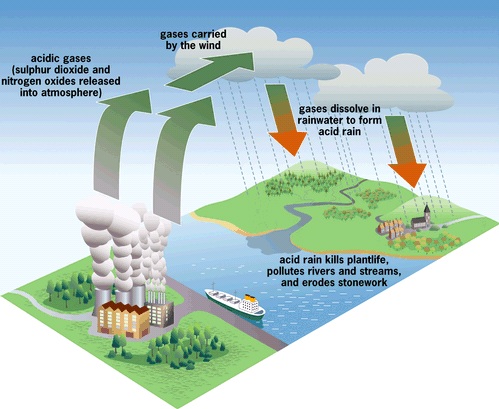
High in the sky, water in the clouds is able to act as a means of coaxing forcing airborne pollutants, such as sulfur dioxide, to become active aqueous constituents (as acid rain). Researchers from the University of Oregon claim that this behavior should be taken into account and included in scenarios based on climate simulations currently being carried out.
The role of sulfur dioxide - a pollutant originating from volcanic gases and many burning processes - in the formation of acid rain is known, but the exact mechanism by which sulfur dioxide reacts on the surface of aqueous particles in the atmosphere to form acid rain is far from being understood.
Chemists from the University of Oregon studied the behavior of sulfur dioxide from the moment it arrives and adsorbs into water at low temperatures, a situation that simulates the conditions prevailing in the atmosphere. Using short-pulse laser beams of visible and infrared light, the researchers were able to track the interactions between sulfur dioxide and water after it reaches the surface of the water.
The results, published in the scientific journal Journal of the American Chemical Society, show that as soon as the sulfur dioxide molecules approach the surface of the water, they are captured by the water molecules on the surface, a behavior that increases in cold temperatures.
Although this "reaching out" provides a possible route for the entry of sulfur dioxide into the aqueous solution, the weak nature of the surface-binding interactions does not guarantee that the behavior of the water molecules will actually help.
"We found that the binding process of sulfur dioxide to the surface of the water is a very reversible situation and it does not always allow the expected entrance," explains one of the researchers. "For example, for water with high acidity, the sulfur dioxide approaches and binds before the surface of the water, but it does not enter deeper into the water."
The degree of absorption of gases, such as sulfur dioxide, has important implications in understanding the behavior of airborne pollutants and their contribution to global warming and climate change. Sulfur dioxide, which dissolves in water, becomes watery and reflects light coming from the ground, while carbon dioxide that accumulates in the atmosphere captures the heat and leaves it on Earth. Understanding the interrelationships between water molecules found on their surface, such as those found in clouds and fog, and pollutants that rise up from human sources to the surface of the ground may help scientists better predict the potential of chemical reactions occurring in the atmosphere and their consequences on the world's climate.
"In the past, we believed that most of the chemistry in the atmosphere occurs when gas molecules collide and react," explains the lead researcher. "Our research is one of the first to provide molecular insights into what happens when an important atmospheric gas, such as sulfur dioxide, collides with the surface of water, and the role of the water itself in encouraging increased activity."
