Researchers are exploring a new way to selectively destroy cancer cells by attaching antibodies from cancer sites to tiny carbon tubes that are heated by exposure to near-infrared light
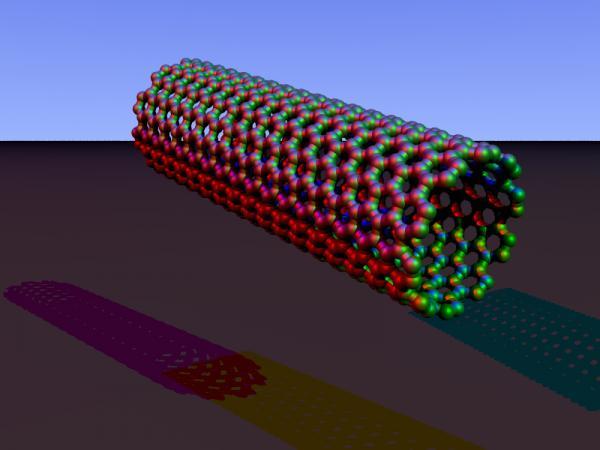
Biomedical scientists from UT Southwestern Medical Center of the University of Texas and nanotechnology experts from UT Dallas describe their experiments in research available in the journal Proceedings of the National Academy of Sciences. Scientists are able to use biological compounds called monoclonal antibodies that bind to cancer cells. These antibodies can act alone or bind to strong anti-cancer drugs, radioactive atoms or toxins in order to transfer the deadly substances to the cancer cells.
In this study, the researchers used monoclonal antibodies that selectively target specific sites on lymphoma cells (cancer of the lymph nodes) to coat them in tiny structures called carbon nanotubes. Carbon nanotubes are extremely small tubes of graphite that heat up when exposed to near-infrared light. This type of radiation, invisible to the human eye, is used in a television remote control to switch between different channels and can be detected by night vision devices. This type of beam can penetrate through human tissue about four centimeters. In cultures of lymphoma cancer cells, the antibody-coated nanotubes bind to the surface of the cells. When the target cells were then exposed to near-infrared radiation, the nanotubes heated up and generated enough heat to "cook" the cancer cells and destroy them. Nanotubes coated with other antibodies did not bind to the cancer cells and therefore also failed to destroy them.
"Using near-infrared radiation to obtain lethal hyperthermia is particularly effective because human tissues do not strongly absorb radiation in this range," says Dr. Ellen Vitetta, director of the Cancer Biological Immunology Center at UT Southwestern and senior author of the paper. "Once the nanotubes bind to the cancer cells, an external source of near-infrared radiation can be used for safe penetration through healthy tissues and elimination of the cancer cells.
"The demonstration of such selective elimination was the goal of the present study. We have already worked in the past for many years with targeted healing methods and even if such a high level of selectivity can be demonstrated in a laboratory plate, there are still many bumps in the transfer of these new methods to clinical studies. We have only just begun to test this with mice, and although there is no certainty that it will work, we are very optimistic."
The use of carbon nanotubes to eliminate cancer cells by local heat has been tested by several research groups, but the new study is the first to show that both the antibodies and the nanotubes retain their physical properties and capabilities - selective binding and elimination of the target cells only. The method also worked properly when the antibody-nanotube assembly was placed in an array designed to mimic the conditions inside the human body. Biomedical applications of nanoparticles are increasingly attracting the attention of both clinical and basic research scientists. However, there are quite a few challenges in the successful development of nanomedical materials. One of them is the possibility that the new nanomaterial could harm healthy cells and organs. This requires an in-depth and careful examination of the effects of nanomaterials on the human body in order to assess their toxicity. "There are comprehensive approaches to identify and minimize indiscriminate toxicity of the nanomaterials developed in our research," says Dr. Rockford Draper, head of the research team at UT Dallas and professor of molecular biology.

4 תגובות
I wish I could, in Israel we are indeed a high-tech powerhouse, but in the field of nanotechnology and bioinformatics there are not that many companies that deal in the field.
And I say this from experience, I chose to deal with computers instead of computational biology because in Israel (except for research in universities) it is not easy to develop ideas like that.
unfortunately.
Eli.R
There is probably no such danger, but if there is, it will be discovered in the experiment.
Of course, there are also other ways to solve the problem and this does not mean that the discussed way should not be tried.
It reminds me of a joke about a mother-in-law who was always arguing with her son-in-law and one day she decided to reconcile and when she came to the store she brought him two ties.
The groom who wanted to show enthusiasm ran straight to the next room, tied one of the ties in front of the mirror and came back to show everyone that he was already wearing it.
The mother-in-law who saw him straight away said: "What is this? And you don't like the other one anymore?"
This. So you are invited to conduct a study that uses P53 and apoptosis.
These researchers will continue their research and others will study other things.
An interesting book in Nasheer is "The Changed Cell" by Steven Rosenberg.
Creating ambient heat can also destroy healthy lymph cells.
There is perhaps no safer way, such as trying to activate the cell's apoptosis mechanism, on the assumption that we know exactly how a T lymphocyte (cytokine) causes the cell's own apoptosis - there is no way to imitate the same mechanism, let's say if we identify a nano molecule that imitates the action of perforin to create apoptosis instead Environmental-specific heating?
Thought-provoking and awe-inspiring.