Richard Heck, Ichi Nigashi and Akira Suzuki share the 2010 Nobel Prize in Chemistry for developing a more efficient way to join carbon atoms together to build molecules that improve our daily lives including the development of cancer drugs, materials for the electronics industry, and more

The Nobel Prize Committee announced today the three winners of the Nobel Prize in Chemistry for 2010. The three winners are: Professor Richard F. Heck from the USA; Professor Ei-ichi Negishi from Japan; Professor Akira Suzuki (Akira Suzuki) from Japan.
A powerful tool for chemists
The existing need for complex chemicals is growing. Humanity needs new drugs capable of curing cancer and preventing the dangerous effects of deadly viruses in the human body. The electronics industry is looking for new materials capable of emitting light, and the agricultural industry is interested in developing materials capable of protecting the crops that are important to man. The 2010 Nobel Prize in Chemistry is awarded to a scientific tool that has improved the ability of chemists to meet all these requirements in the most efficient manner: palladium-catalyzed cross coupling.
In the late 33s, divers in the Caribbean Sea found the marine sponge Discodermia dissolute. At a depth of XNUMX meters they discovered a creature without eyes, mouth, stomach and bones. At first glance, the creature looks primitive, but its and other sponges' ability to drive away their marine enemies has made them a magnet for chemists. They have an amazing ability to produce complex and large chemical secretions that serve as poison and keep other organisms away from attacking and exploiting them.
Researchers have discovered that many of these toxins have medicinal properties; They can be used as antibacterial, antiviral and even anti-inflammatory drugs. In the case of the marine sponge Discodermia dissolute, the first laboratory experiments showed that an active substance found in the contents (discodermolide) could be used in the future as a chemotherapy drug. Among its other properties, it is able to prevent the division of cancer cells in laboratory cultures.
Getting out of the way from a serious teacher in the face of scientific progress
Following more detailed studies, scientists were able to show how the substance extracted from the sponge destroys cancer cells in the same way that the drug Taxol works, one of the most common anti-cancer drugs around the world today. Finding a substance with such amazing ability is a satisfying experience in itself, but in the absence of the discoveries for which the Nobel Prize in Chemistry was awarded in 2010, the story of this substance would have ended here. Progress was supposed to stop here due to a lack of the active substance, since it is not possible to develop medicines based on a substance found in only small quantities in the depths of the Caribbean Sea. However, thanks to the addition of the scientific means of palladium-catalyzed cross-coupling reactions developed by chemists Richard F. Heck, Ei-ichi Negishi and Akira Suzuki, scientists are now able to prepare Synthetically the active ingredient so important discodermolide. One form of the Geschi reaction was used as a major step in the synthesis of this material. Other scientists were able, as a result, to improve the process and obtain sufficient quantities of the material to begin clinical tests in humans with cancer.
Only in the future will we know if this substance will lead to the development of a life-saving drug. In any case, this is just one example of the way in which substances found in nature inspire chemists. What is common to all molecules found in living organisms, what is known as organic molecules, is the fact that they all consist, more or less, of a complicated skeleton of carbon atoms. Carbon-carbon bonds are the basis of the chemistry of life itself, and their importance among chemists is faithfully represented by the fact that five Nobel prizes in chemistry have been awarded to date on this research topic. The previous four awards are: Grignard reaction (1912), Diels-Alder reaction (1950), Wittig reaction (1979) and Olfini metathesis reaction (2005).
Palladium - the meeting point of the carbon atoms
Palladium-catalyzed cross-coupling reactions are unique in that they can be performed under mild conditions and with great accuracy. Previously, chemists had to initiate the chemical reaction between two carbon atoms using active substances. Such substances do fulfill their purpose, but due to their high activity they also lead to obtaining unwanted by-products that originate from the reaction of the carbon atoms with other atoms. When chemists want to make large preparations such as discodermolide, they build the preparation in several steps, step by step. If the amounts of the unwanted by-products are too large, then a sufficient amount of the desired substance will not be obtained and the reaction itself will be ineffective and even too expensive.
In the palladium-catalyzed reaction, scientists use the metallic element palladium as the meeting point for the carbon atoms. The carbon atoms bind to the palladium atom, and thus they get close enough to each other to initiate the start of the reaction between them. The palladium atom is used as a chemical catalyst - a catalyst. He takes part in the process, speeds it up, but he himself does not perish during it.
The impact of industrial progress
The possibility of using palladium as a catalyst began to lead to interest during the XNUMXs. At this time a German chemical company, Wacker Chemie AG, began using palladium to convert ethylene to acetaldehyde - an important raw material used in adhesives, plastic softeners and in the production of acetic acid.
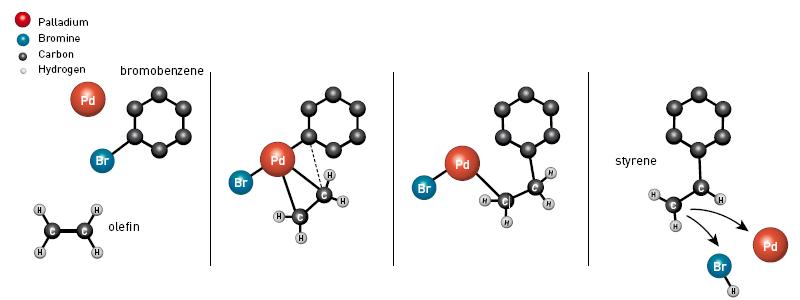
Richard Huck was working for an American chemical company in Delaware, and while the chemical industry was becoming increasingly intrigued by the successful Wacker process, he began conducting experiments using palladium as a catalyst. In 1968 he published his successful research through a series of scientific articles. Among his other findings, he was able to connect a ring of carbon atoms to a shorter unit of carbon to obtain the important material styrene (picture 2) - a main component of the plastic material polystyrene. After four years he further improved his reaction and today the reaction known as the Heck reaction after his name is one of the most important reactions in the creation of single bonds between carbon atoms. It is used, for example, in the commercial-scale production of the anti-inflammatory drug naproxen, in the anti-shortening drug montelukast and in the production of an important material in the electronics industry.
The number eight is a magic number in organic chemistry
In order to understand the importance of Richard Hack's discovery we must dive into the world of atoms; into the "cloud" of electrons surrounding the nuclei of atoms. Electrons are often depicted as tiny particles orbiting around the atomic nucleus. But the electron is more like a negatively charged cloud surrounding the positively charged nucleus.
Around the atomic nucleus there are several layers of electron "clouds" and the larger the atom, the greater the number of layers. Chemists are interested in the number of electrons found in the outermost layer, since all chemical reactions originate from the "need" of the atom to fill this layer. The small atoms, essential to organic chemistry, i.e. carbon, oxygen and nitrogen, should always have eight electrons in this outer layer. Eight is a magic number in organic chemistry.
In its original form, the carbon atom has only four electrons in the outer shell. Therefore, it tends to combine with other atoms so that the electrons are shared together in the form of chemical bonds. For example, in the simplest organic compound, methane, carbon shares electrons with four other hydrogen atoms. In this way the outermost layer is filled as required and the atom achieves its goal.
Rowing in full steam
When chemists synthesize complex prodrugs such as discodermolide, they take a shortcut and utilize existing smaller prodrugs as building blocks. However, connecting these small pieces is not so simple. The carbon atoms in the smaller atom already share their electrons; They already have eight electrons in the outermost shell and are essentially stable now. They have no reason to react with a carbon atom in another molecule.
The chemist's task at this stage is to excite the carbon atom and force it to be more inclined to react with another carbon atom. Victor Grignard, winner of the Nobel Prize in Chemistry in 1912, found a solution to this problem. Through several chemical tricks he attached a magnesium atom to a carbon atom which he wanted to make more active. Magnesium has two electrons in its outer shell, and would rather get rid of them. In a substance known as a Grignard reagent, the magnesium atom transfers its two electrons toward the bond so that they are closer to the carbon atom. As a result, electrons are added to the outer electron shell of the carbon atom, but at the same time an imbalance is created between the positively charged nucleus of the atom and its negatively charged electron cloud. The carbon atom becomes less stable, and as a result "looks" for another atom with which it can bond.
Accuracy - the key to building huge furrows
The Grignard method for coupling carbon atoms is very important in chemistry. However, when dealing with the preparation of complex and large fridos, the method has its own drawbacks. The unstable carbon atom in the Grignard reagent does not behave as expected. When the reactant encounters several different carbon atoms with which it can react, too many unwanted by-products are formed for the reaction to be effective.
A palladium-catalyzed cross-coupling reaction solves this problem and gives more precision to the process. When carbon atoms encounter a palladium atom, chemists do not need to activate the carbon atom to the same level. This fact causes by-products to be obtained in a smaller amount and to increase the efficiency of the reaction.
Instead of the Grignard reagent, Richard Hack began using chemical compounds called olefins. In this compound, the carbon atom is initially activated somewhat and when it binds to the palladium atom it becomes even more active and its chance to react with another atom increases.
In 1977, Hei-ichi Nigashi developed a slightly different form of Grignard reagent when he replaced the magnesium with a zinc atom. The carbon becomes less active when zinc is used, but the zinc transfers the carbon atom to the palladium atom. When the carbon atom gets closer to another carbon atom attached to the palladium atom, they are more likely to bond with each other.
Two years later, Akira Suzuki began using a pit foundation. This atom is the mildest activation factor to date and is even less toxic than zinc, a fact that is an advantage when production reaches a commercial level. For example, the Suzuki reaction is used in the commercial preparation (thousands of tons) of an active substance that protects agricultural crops from a harmful fungus.
Today, the Haack reaction, the Gishchi reaction and the Suzuki reaction are of great importance to chemists. One of the most spectacular examples for the use of a palladium-catalyzed cross-coupling reaction is the preparation of the substance palytoxin - a giant in the chemical world. It is a natural poison that was first isolated from corals in Hawaii in 1971. The substance contains 129 carbon atoms, 223 hydrogen atoms, 3 nitrogen atoms and 54 oxygen atoms. In 1994, scientists were able to prepare this giant particle, partly with the help of the Suzuki reaction.
Very challenging syntheses, such as that of palytoxin, force chemists to improve and optimize their means. Moreover, it is important to artificially synthesize natural materials in the laboratory for research purposes. When scientists discover a new substance they use different chemical methods to understand how the different atoms are arranged in it. However, the only way to verify this structure is through artificial synthesis and comparing it to the natural substance.
A means of searching for new drugs
As demonstrated above, the palladium-catalyzed cross-coupling reaction is an important tool in the search for new drugs. Today, scientists around the world use the oceans as a huge "pharmacy". They managed to isolate thousands of substances from organisms living in the sea, and these substances provided an incentive for further scientific progress. Except for the substance discodermolide, the substance diazonamide A, which originates in the Philippines, was prepared in a similar synthetic process. In laboratory experiments, this substance has been shown to be effective against colon cancer. Another example is the substance dragmacidin F, which was isolated from a sponge found on the coast of Italy. Preliminary laboratory experiments showed that this substance is effective against herpes viruses and AIDS.
Chemists also use this reaction to modify natural medicinal substances in order to improve their effectiveness. An example of this is the substance vancomycin, an antibiotic substance that was first isolated in the XNUMXs from a soil sample taken from the jungles of Borneo. Today, this substance is used against staphylococcus bacteria of species that have developed resistance to most of the antibiotics that are common today. Naturally, these bacteria are harmless, but they can infect wounds and cause complications after organ transplantation. Due to this dangerous development, scientists are trying to introduce changes to the structure of the substance so that it can also act on the strains resistant to it. Using the successful chemical means they prepared strains of the substance that were effective against these resistant species.
Even thinner computer monitors
The electronics industry also takes advantage of the palladium-catalyzed cross-coupling reaction, for example when looking for better radiation sources for diodes. Organic light emitting diodes (OLEDs) contain organic particles that emit light. They are used in the electronics industry in the production of extremely thin displays, with a thickness of a few millimeters. Chemists used the reaction to enhance the blue light emitted by these diodes.
Infinite progress
Since the Hack reaction, the Suzuki reaction, and the Gishchi reaction are all extremely important in the preparation of complex chemicals, other chemists have also improved and modified these reactions. One such change has to do with this year's Nobel laureate in physics. In the spring of 2010, scientists reported that they had succeeded in attaching palladium atoms to the material graphene, and the resulting solid was then used to perform the Suzuki reaction in water.
The palladium-catalyzed cross-coupling reaction is still used as a basis for development and research, even after 40 years have passed since Richard Hack began his experiments in the Delaware laboratories. The discoveries of the three chemists already today are of great importance to all of humanity. At the same time, in light of the developments taking place today in laboratories around the world, it is likely that their reactions will become even more important in the future.
Material for further reading
Scientific articles
Heck, RF and Nolley, JP (1972) J. Org. Chem. 37, p. 2320.
Negishi, E.-I., King, AO and Okukado, N. (1977) J. Org. Chem. 42, p. 1821.
Miyaura, N. and Suzuki, A. (1979), J. Chem. Soc. Chem. Commun., p 866.
Review articles
Negishi, E. (1999) A profile of Professor Richard F. Heck Discovery of the Heck reaction. Journal of Organometallic Chemistry 576, p. XV-XVI.
Rouhi, M. (2004) Chem. & Eng. News, 82 (36), Sept. 6, p. 49–58. [Article about Suzuki.]
de Meijere, A. and Diederich, F. (Eds.) (2004) Metal-Catalyzed Cross-Coupling Reactions, vol. 1 and 2, Wiley-VCH, Weinheim. pp. 916.
Buchwald, SL (Ed.) (2008) Accounts of Chemical Research, Vol. 41, Nov. 11, p. 1439–1564. [Special issue on Cross Coupling.]

4 תגובות
Written eloquently and interestingly. Equal to everyone, but also of interest to those who understand the field
Good and interesting article, congratulations.
point,
It sounds like you don't know what you're talking about.
It sounds like they are like those scientists trying to develop an efficient way to win a Nobel Prize.