There is nothing to argue about taste and smell. But should you know how the body senses them? The answer: G protein-coupled receptors, the subject of the 2012 Nobel Prize in Chemistry

The Nobel Prize in Chemistry for 2012
The 2012 Nobel Prize in Chemistry was jointly awarded to Robert J. Lefkowitz and Brian K. Kobilka, two scientists from the USA, for their research on G protein-coupled receptors.
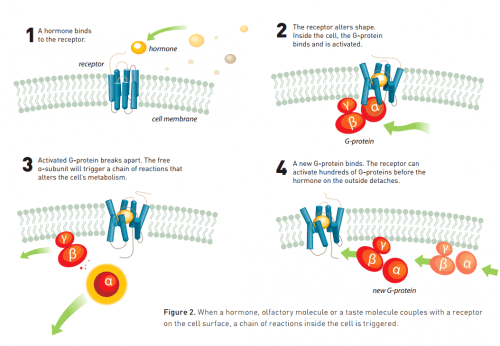
G protein-coupled receptors are receptors that belong to a large family of transmembrane proteins (transmembrane proteins), which are stimulated by molecules outside the cell. The stimulus causes a chain of chemical reactions inside the cell that are activated by the receptor. Ultimately, the process causes a cellular response called a signal transduction pathway. G protein-coupled receptors are found exclusively in eukaryotic cells, which include yeast, plants and animals. The ligands that bind to the receptors and activate them can be light-sensitive compounds, odors, pheromones, hormones, neurotransmitters, etc. The ligands can be different in size - from very small molecules to large polypeptides. G protein-coupled receptors are the target of many modern drugs due to their involvement in many diseases.
cells and sensing
In our eyes, in our noses, there are sensors for light, smells and tastes. Inside the body, cells have similar sensors for hormones and other signaling substances, such as adrenaline, serotonin, histamine and dopamine. With the development of life, cells repeatedly used the same basic mechanism to sense their environment: G-protein-coupled receptors. However, these remained hidden from the researchers for a long time.
In the drum of the human body thousands of billions of cells communicate with each other. Many of them have developed distinct roles for themselves. Some store fat; Others sense visual signals, produce hormones or build muscle tissue. In order for us to function properly, it is essential that our cells work together, in coordination, so that they can sense their surroundings and understand what is happening there. For this they need sensors.
Sensors located on the surface of the cell surface are called receptors. Robert J. Lefkowitz and Brian K. Kobilka were awarded the 2012 Nobel Prize in Chemistry for mapping how a family of receptors known as G protein-coupled receptors (GPCRs) work. In this family you can find receptors for adrenaline (epinephrine), dopamine, serotonin, light, taste and smell. Most of the physiological processes occurring in the body depend on these receptors. About half of the drugs today act on these receptors, including beta blockers, antihistamines and many types of psychiatric drugs. Therefore, gaining knowledge about this family of receptors should bring benefit to the human race. At the same time, these receptors escaped the notice of the scientists for a long time.
The receptor - an elusive enigma
At the end of the 19th century, while scientists began to conduct experiments about the effects of adrenaline on the body, they discovered that this substance causes an increase in heart rate and blood pressure and slows down the activity of the pupils. Since the researchers suspected that the adrenaline travels through the nerves in the body, they began to paralyze the nervous system of laboratory animals. Despite this, the activity of adrenaline continued. Their conclusion was: the cells must contain a certain type of receptors that allow them to sense chemical substances - hormones, poisons and drugs - in their environment.
However, when the researchers started looking for these receptors, they ran into a wall in the form. They wanted to understand what these receptors look like and how they transmit the signals to the cell. Adrenaline was injected outside the cell and this led to its metabolism which could be measured inside the cell. Each cell has a wall: a membrane of fat molecules that separates it from its environment. How does the signal get through this barrier? How does the inside of the cell know what is happening outside the cell?
The receptors remained undeciphered for decades. Despite this, scientists have managed to develop drugs that affect these receptors in a specific way. In the XNUMXs, the American scientist Raymond Ahlquist studied how different organs react to a variety of adrenaline-like substances. His research led him to the conclusion that there must be two types of adrenaline receptors: one type that causes the muscle cells in the blood vessels to contract, and a second type that is mainly responsible for the excitation of the heart. He named the two receptors alpha and beta. Soon after, scientists developed the first beta blockers, which are today some of the most effective drugs for heart disease.
These drugs undoubtedly affect the cells, but the exact mechanism of their activity remains unknown. Now we know why it was so difficult to find the receptors: their number is relatively few and most of them are hidden inside the cell wall. After several decades, even Raymond Ahlquist began to give up on his theory regarding the two types of receptors, saying: "For me, they are an abstract idea formulated to explain the observed response of tissues following their contact with chemicals of different structures."
At this point, in the late 2012s, Robert Lebkovitch, one of the XNUMX Nobel Prize laureates in chemistry, entered into the history of these receptors.
Lure the receptors out of hiding
The outstanding young student decided to become a cardiologist. However, he completed his undergraduate degree during the Vietnam War and did his military service in the US Public Health Service at the Federal Research Institute of the National Institutes of Health. There he was presented with the great challenge of finding the receptors.
Levkovic's supervisor already had a plan ready. He suggested adding a radioactive iodine atom to the hormone. Then, once the hormone binds to the surface of the cell, it will be possible to follow the radiation emitted from the iodine and locate the receptor. In addition, in order to further strengthen the results, Levkovitch had to prove that it is indeed the binding of the hormone from the outside of the cell that is responsible for initiating the process that takes place inside the cell. If he succeeds in this, no one will be able to doubt that he has in fact discovered the receptor with the biological role.
Levkovic began working with a hormone that stimulates the production of adrenaline in the adrenal gland. But nothing seemed to work for him. The years passed and he was still unable to make progress in his research, and Levkovic, who was not enthusiastic about doing this research in the first place, began to despair. Although he continued his research, he still aspired to become a doctor.
In his second year of research, Levkovic finally began to make some progress. In 1970 he published articles in two prestigious journals (PNAS, Science) in which he described the discovery of an active receptor. Following this publication, he came to Duke University in North Carolina. There, in his new laboratories, Levkovitch organized his own team of researchers. Although he realized he would no longer be a cardiologist, he still wanted to study heart disease. And so he began to focus on receptors for adrenaline and noradrenaline, substances called adrenergic receptors. Using radioactively labeled substances, including beta blockers, his research group examined how these receptors work. And after sharpening their tools, they finally succeeded, thanks to their high skills, in extracting several receptors from biological tissue.
Meanwhile, knowledge about the mechanism operating inside the cells expanded. Researchers discovered the receptors called G-proteins (for which the Nobel Prize in Physiology and Medicine was awarded in 1994) that are activated by a signal originating from the receptor. This protein, in turn, activates a chain of reactions that affects the metabolism of the cell. Starting in the XNUMXs, scientists began to gain insights into the process by which signals are transmitted from the outside of the cell to the inside.
The garden - the key to new insights
In the XNUMXs Lebkovitch decided that his research group needed to find the gene that codes for the beta receptor. This decision turned out to be critical to Levkovic's success. A gene is similar to a fingerprint - it contains a code that is read by the cell while connecting amino acids to form a protein, for example - a receptor. The idea was that if the research group succeeded in isolating the gene and deciphering the fingerprint for building the beta receptor, the researchers would be able to gain insights into how the receptor works.
Around the same time, Levkovic hired a young doctor, Brian Kovilka. His curiosity about the adrenergic receptors was born out of his experience in the intensive care unit of the hospital. A shot of adrenaline can be the thin line between life and death. This hormone opens the blocked respiratory system and speeds up the heart rate. Kobilka was interested in testing the nature of epinephrine at the most detailed molecular level, so he turned to Levkovic's laboratory.
Kobilka began the hunt for the garden. However, in the XNUMXs trying to find a single gene out of the body's vast sea of genes was like finding a needle in a haystack; The technical challenge slowed research progress. However, Kovilka had an ingenious idea that would allow the isolation of the gene. With eager anticipation, researchers began examining the cipher; They discovered that the receptor consists of seven long, fatty (hydrophobic) helical strands - what is known as the helix. This structure suggests to the researchers that the receptor likely moves in and out of the cell wall seven times.
seven times. It was the same number of strands with the same helical shape of another receptor that had already been discovered elsewhere in the body: the light receptor rhodopsin found in the retina of the eye. At this point an idea was born: are these two receptors related, even though they have completely different activities?
Robert Lebkovitch described this moment as "a real eureka moment". He knew that both the adrenergic receptors and the rhodopsin receptor come into contact with G-proteins that are inside the cell. He also knew that nearly 30 other receptors function through G-proteins. The obvious conclusion: there must be a whole family of receptors that look similar and work in a similar way!
Since this breakthrough discovery, the complex has slowly taken shape and scientists now have the detailed knowledge of G protein-coupled receptors – how they work and how they regulate molecular activity. Levkovic and Kovilka were at the forefront of this scientific journey and last year, in 2011, Kovilka and his team of researchers reported a finding that was the highlight of their work.
Simulating the effects of adrenaline
After successfully isolating said gene, Brian Kovilka transferred to Stanford University School of Medicine in California. There he prepared to create the image of the receptor - an impossible goal in the eyes of most scientists - and for Kobilka, it became a long journey.
Protein imaging is a process that involves very complex steps. The proteins are too tiny to be distinguished under a normal microscope. Therefore, the scientists make use of a method known as X-ray crystallography. With this method, one begins by creating a crystal of the protein, a structure in which the proteins are densely packed in a symmetrical and orderly manner, similar to the water molecules packed in an ice crystal or the carbon atoms in a diamond. Next, the scientists beam X-rays through the crystalline protein. When the rays hit the proteins they splash out. From this entanglement image the scientists are able to deduce the detailed atomic structure of the protein.
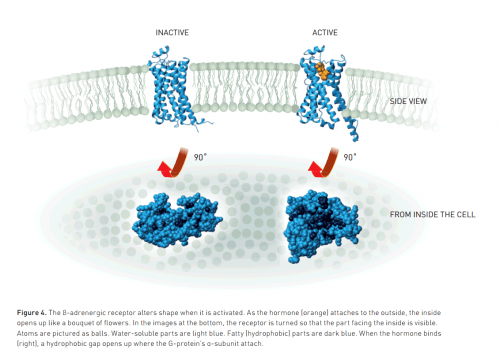
The first image of a crystal structure of a protein was obtained in the XNUMXs. Since then, scientists have used this method to create thousands of images of proteins. However, most of them were water-soluble, a property that facilitates their formation process. Fewer researchers have been able to simulate proteins located in the lipid membrane of the cell. In water, such proteins dissolve poorly, similar to a drop of oil on water, and form greasy lumps. Moreover, G protein-coupled receptors are inherently very mobile (they transmit signals by movement), but within a crystal structure they must remain almost stationary. Their formation, therefore, is a serious challenge.
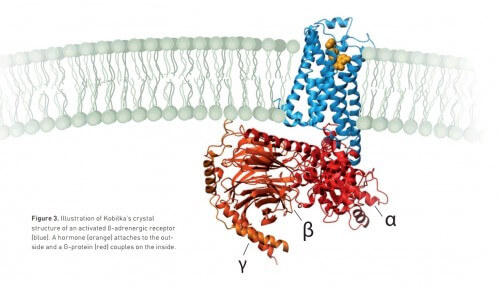
Only two decades later Kobilka was able to find the solutions to all these problems. And thanks to determination, creativity and the methods of molecular biology, Kobilka and his research group finally achieved their goal in 2011: they got a picture of the receptor at the very moment it transmits the signal from the hormone outside the cell to the G protein inside the cell.
The image, published in the prestigious scientific journal Nature, reveals new details about G protein-coupled receptors, for example, what the activated receptor looks like once it opens a gap to which the G protein binds. Such knowledge will be very useful in the future for the development of new drugs.
Life needs flexibility
The mapping of the human genome has revealed close to a thousand genes that encode G protein-coupled receptors. About half of these receptors respond to odors and are part of the olfactory system. About a third of them are receptors for hormones and signaling substances, such as dopamine, serotonin, prostaglandin, glucagon and histamine. Several receptors capture the light rays that hit the eye, while others are located on the top of the tongue and give us the sense of taste. And yet, over a hundred receptors pose a challenge to scientists, since their roles have not yet been clarified.
Beyond the discovery of the different versions of the receptor, various researchers, led by Levkovic and Kovilka, discovered that they are multifunctional; One receptor can recognize several different hormones that are outside the cell. Moreover, in the interior of the cell they do not only react with the G proteins, but they also react, for example, with proteins called arrestins. The realization that these receptors are not always coupled to G-proteins led the researchers to refer to them as transmembrane receptors 7 (7TM), after the seven helical strands included in them.
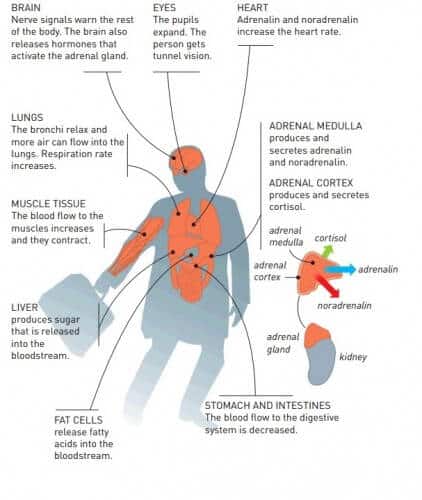
The number and flexibility of the receptors allow the fine regulation that is so essential to the functioning of life. When we encounter a fearful situation, our blood is filled with adrenaline and different tissues react in different ways. Blood flow to the digestive organs decreases; At the same time, the flow to the muscles increases. These different results of adrenaline depend on the presence of nine different receptors that react with the same hormone. Some receptors initiate cell activity, while others have calming effects.
