Edible compounds containing sugar, salt and alcohol can effectively detect, capture and store carbon dioxide
About a year ago, chemists from Northwestern University published their recipe for making a new set of nanostructures composed of sugar, salt and alcohol. Now, the same team has discovered that these edible compounds can effectively detect, capture and store carbon dioxide.
The porous crystals - known by their scientific name "metal-organic frameworks, MOFs" - include only natural components and are simple to prepare, a fact that makes them have a huge advantage compared to other metal-organic frameworks. Ordinary materials from this family, which are also effective in absorbing carbon dioxide, are usually produced from materials based on crude oil and sometimes they also include toxic heavy metals. Other characteristics of metal-organic frameworks are their ability to appear red when completely filled with carbon dioxide, and the reversibility of the trapping process. The research findings were published in the scientific journal Journal of the American Chemical Society.
"We can take molecules derived from atmospheric carbon, through the process of photosynthesis, and use them to capture an additional amount of carbon dioxide," says Ross S. Forgan, one of the researchers who co-authored the paper. "Thanks to the preparation of our materials from natural materials, not only do we produce materials that are completely non-toxic, but we also reduce the amount of carbon dioxide gas emissions involved in their production." The main component, gamma-cyclodextrin, is a biologically renewable sugar molecule produced from corn starch. The sugar molecules are anchored in place by metal atoms derived from salts such as potassium benzoate or rubidium hydroxide, and the precise arrangement of the sugars in the crystal is what gives them the ability to successfully capture carbon dioxide.
"It turns out that a rather unexpected event occurs when many sugar molecules are placed next to each other in a basic environment - they begin to react with carbon dioxide in a reaction similar to oxygen fixation, which is the process by which sugars are produced in nature in the first place," notes the lead researcher. "As a result of this reaction, the carbon dioxide is tightly bound inside the crystals, but it can still be easily released afterwards."
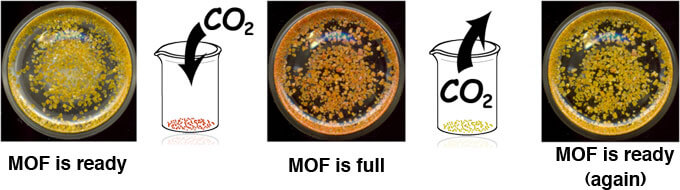
The fact that carbon dioxide reacts with the metal-organic frameworks, an unusual occurrence, led to the development of a simple method to identify the moment when these materials reached their full capacity. The researchers placed an indicator molecule, which detects changes in the degree of acidity by changing its color, inside each of the crystals. When the yellow crystals of the substance are filled with carbon dioxide they change their color to red.
The simplicity of the new materials, along with their low cost and environmental friendliness, make them good candidates for future commercialization. Adds one of the researchers: "I believe that our findings are an extraordinary demonstration of the applicability of simple chemistry for contemporary problems such as carbon capture and sensing technologies."

5 תגובות
Ori, you can heat with the help of the sun directly - with the help of mirrors or simply in a warm area, so that it does not emit CO2 in an uncontrolled manner. An effective and cheapest method possible
to an anonymous user.
Doesn't the heating of the material mean the emission of carbon for the purpose of heating?
Just send a tanker to Mars to disperse and transform the atmosphere?
Laurie S.
The process is indeed reversible - the gas can be emptied out (into a collection vessel for example) by heating the material.
The material is emptied of the gas and is ready for a renewed round of absorption.
After capture, how is the carbon dioxide "emptied" and where? And is the compound ready for action again?