Scientists have been trying for many years to find materials that can effectively capture carbon. New research demonstrates how chemical changes affect the ability of buckeye balls to store greenhouse gases.
[Translation by Dr. Nachmani Moshe]
Scientists have been trying for many years to find materials that can effectively capture carbon. New research demonstrates how chemical changes affect the ability of buckeye balls to store greenhouse gases.
Scientists from the laboratory of chemist Andrew Barron at Rice University They discovered the previous year that carbon-60 type molecules (Bucky balls discovered at the same university in the 60s) are able to store carbon dioxide when combined with a polymer called polyethylene imine (Wikipedia, PEI). The research findings have long been published in the scientific journal Energy and Fuels. The amine group-rich combination of the buckeye balls (CXNUMX) and the PEI polymer has shown its ability, in a previous study, to capture emissions of carbon dioxide which is a greenhouse gas from various sources such as chimneys of industrial plants and natural gas drilling.
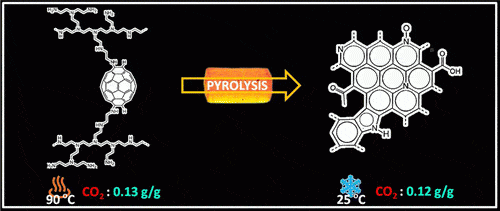
During the new study published in July 2015, the researchers found that heating the materials in an oxygen-free environment (pyrolysis) caused changes in the chemical composition, changes that increased their ability to capture carbon dioxide. "One of the things we wanted to know was exactly at what point the materials are able to trap the maximum amount while the temperature is the lowest possible," said the lead researcher. The researchers performed the pyrolysis process in a temperature range of 1000-100 degrees Celsius and checked the amount that was captured in the materials. They found that the optimum point is 200 degrees Celsius. The material pyrolyzed at low temperatures became sticky and viscous and failed to capture carbon dioxide from high temperature sources. The researchers found the opposite finding when the pyrolyzed material was the combination of PEI-C60. The fragile, now porous material was found to be more effective in low temperature environments at trapping carbon dioxide molecules. The researchers found that at a temperature of 200 degrees Celsius, the heat treatment breaks down the chemical bonds in the polymer between carbon and nitrogen, which leads to a significant reduction in its ability to capture carbon dioxide.
The researchers found that at its peak, the untreated PEI-C60 blend absorbed more than a tenth of its weight in carbon dioxide at high temperatures (0.13 grams per gram of material at 90 degrees Celsius). The treated PEI-C60 blend (pyrolyzed) absorbed carbon dioxide in a similar amount at low temperatures (0.12 grams per gram of material at 25°C). The researchers, focused on obtaining possible benefits for environmental conservation, continue to improve and optimize their process.
