An artificial intelligence model sheds light on the specialization pathway of muscle cells and reveals an important control node along the way
The life sciences have never been more digital. Biologists collect enormous amounts of data and sophisticated computational models are required to decipher them and learn something new about life processes. Dr Uri Avinaam from the Department of Biomolecular Sciences at the Weizmann Institute of Science has faced an unsolved biological question in recent years - how do new muscle fibers form from stem cells? In search of answers, he shared the problem with his friend - Dr Assaf Zaritsky from the Department of Software and Information Systems Engineering at Ben-Gurion University of the Negev - and together they began to develop a machine learning model that could decipher the complex biological process. The discoveries of the model are presented in an article recently published in the scientific journal Molecular Systems Biology and show that, surprisingly, when building a muscle, each stem cell goes through a maturation process unique to it.
The stem cells from which the muscle tissue develops are formed in the embryo, but a handful of them are preserved in adult muscles. These cells are dormant most of the time, but during growth, intense physical activity or injury they come into action. In the first step, the activated stem cells divide to regenerate the tissue. After that, they stop dividing and undergo "differentiation" - a process of maturation of stem cells in which they specialize in a unique role and acquire properties necessary to perform it. In the case of the muscle, the differentiated stem cells elongate, produce protein fibers that enable the familiar contraction action of the muscles - and migrate to the place where the tissue regenerates. There, a large number of contractile cells join together to form a single, long cell known as a muscle fiber. A collection of such cells constitutes the complete muscle. However, to date scientists have had difficulty understanding how stem cells progress along this specialization pathway and what are the factors that motivate them to move from state to state.
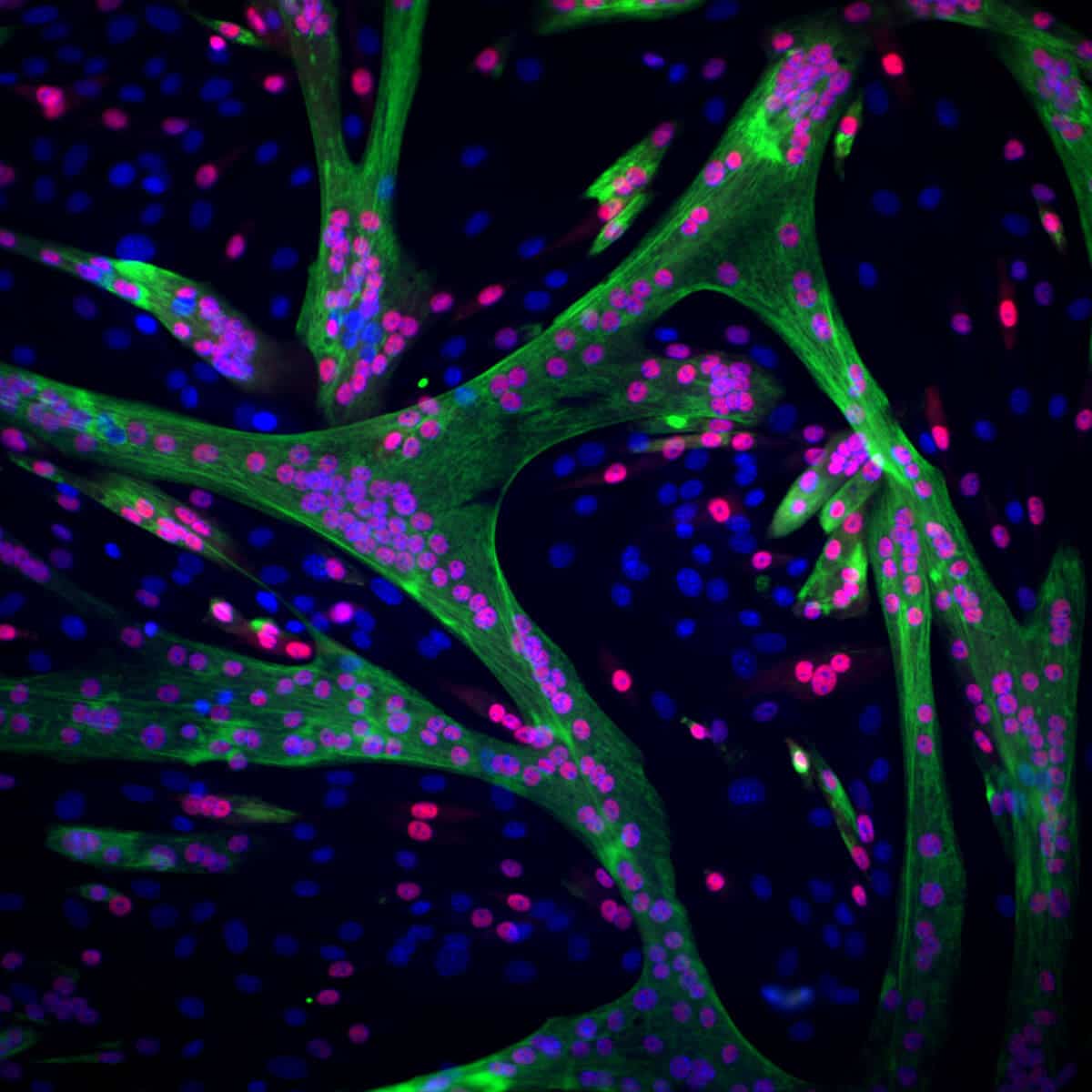
In an attempt to answer the question, Julia Sarfati and Adi Hezek, from Dr. Avinaam's laboratory, documented on "live" how a muscle fiber was built from mouse stem cells. They decided to follow two changes - the movement of the cells in space along the track and the construction of protein fibers within the stem cells, which is essential for the creation of a mature muscle capable of contraction. To follow the movement of the cells, their nuclei were marked with a fluorescent dye, and to follow the construction of the protein fibers, a protein called actin that makes them up was marked. During a day of differentiation, the researchers produced thousands of videos documenting step by step, at the single cell level, how hundreds of stem cells transform into mature muscle cells and assemble into a new fiber.
After collecting the abundance of biological information, the researchers from the institute and research student Amit Shakerchi from Dr. Zaritsky's laboratory began a joint learning process whose goal was to build a model that would be able to follow the dynamic process. "The research groups had to learn each other's language," says Dr. Avinaam. "Assaf's team learned what a sorted muscle cell is and how to know that it has combined with other cells to form a muscle fiber, while my team learned the principles of machine learning and how to analyze information collected from a sequence of observations at different times. Then, we had to figure out together how to translate the biological process into a computational model that would track its progress."
There are many challenges in building a computer model capable of following a dynamic biological process. "First, you have to decide which time intervals to look at and compare between them," explains Dr. Zaritsky. "Then they decide whether to produce a guided learning model that only takes certain data into account or to allow the model to give weight to all the data. We decided on a guided model that follows the movement path of the cells and the intensity of the fluorescent color obtained from their actin fibers. The model also examined derivatives of this information, such as changes in the movement rate of the cells and how the structure of the actin fibers in the cell changes over time."
The scientists recognized that as the differentiation process progressed, the motility of the cells decreased and the strength of the signal received from the actin fibers in them increased. A machine learning model was trained to distinguish between stem cells and mature muscle cells and created a quantitative index that follows in real time and gives a numerical score to each cell in the sample according to its progress in the process. When they tested the model on experiments that were not trained on, they identified that the score received by most of the stem cells increased gradually during differentiation and stopped increasing at the end. "We learned from the model that the differentiation process is gradual and distributed - the cells do not progress together in stages, but have different patterns of progression," says Dr. Avinaam. "This was an unexpected result, we assumed there would be collective behavior."
"The ability to monitor cell changes in real time and continuously, may allow in the future to monitor the progress of diseases in an unprecedented way. Today, for example, cancerous tumors are monitored by taking a sample (biopsy) - a procedure frozen in time that does not allow receiving current information about a dynamic biological process," says Dr. Zaritsky. "Also, in an age where cultured meat is on the table, models of this type can teach us why cells that have arrived from certain specifications multiply and differentiate more efficiently and quickly, and thus improve production methods."
Stop before you get dressed
The model did indeed reveal that cells completed their maturation at different times, but when they finished differentiating, a specified period of time of about three hours passed on average until they united into a muscle fiber. This finding raised the hypothesis that there is a control point, within which the cell verifies that it has indeed reached its end, and only then initiates the fusion process.
Previous studies have suggested that the p38 enzyme regulates muscle building: when its activity is inhibited, cells do not assemble into a new muscle fiber. However, it was not known at what point in the maturation journey the stem cells get stuck and why. When they ran the computer model, the researchers saw that cells in which the enzyme was inhibited received a numerical score that went up. In other words, even in the absence of the enzyme, they were able to complete their differentiation process, but did not proceed to the fusion stage. Therefore, the scientists concluded that the control point comes at the end of the differentiation process and before the fusion stage.
The model also offered a possible explanation for the enzyme's action: it showed that in cells where its action was inhibited, the way the actin fibers were shaped during differentiation changed. An actual examination of the levels of proteins expressed in the cells that were inhibited confirmed the model's prediction: the scientists identified that the cells express high levels of proteins involved in the arrangement of actin fibers in the cell skeleton, an important step in differentiation and preparation for fusion, alongside low levels of proteins involved in the formation of mature muscle fibers and muscle contraction. "The cells actually get stuck in a 'ready to fuse' state," explains Dr. Avinaam, "therefore, when the enzyme is restored to action, they can recombine and fuse." This is actually a central control node where the muscle makes sure that the cells have completed all the necessary preparations before they fuse into a new muscle fiber. Beyond muscle building, this discovery also indicates the ability of computer models to identify important control nodes in dynamic biological processes."
Reut Malam from Ben-Gurion University in the Negev also participated in the study; Karina Hook from the Department of Biomolecular Sciences at the institute; and Dr. Tamar Ziv from the Technion.
More of the topic in Hayadan:
