Stem cell research is one of the "hot" fields in biological and medical research today. After a decade of discoveries that led researchers to a better understanding of the activity of stem cells in the developing fetus and in the body of an adult, we are now on the threshold of a new era in medicine, an era in which we can try to treat patients with a wide variety of diseases that modern medicine still does not know how to deal with
Unlimited potential
Stem cells are cells that have not yet undergone final differentiation processes, that is, cells that still have the potential to become a different type of cell. A few-day-old fetus consists exclusively of stem cells, and later in the pregnancy, when the body's organs begin to be built, their relative proportion becomes smaller and smaller. However, stem cells in different stages of differentiation can be found both in a baby emerging from its mother's womb and in an adult.
Another important characteristic of stem cells is their unlimited ability to divide, which also results from high expression of telomerase. Under suitable conditions, these cells will continue to divide forever and provide generation after generation of stem cells; And under other conditions, found for example in the developing embryo, most of the stem cells differentiate and create specific cells that build the various tissues. After birth, the stem cells continue to serve as a reservoir for the creation of new cells, and they differentiate according to need and replace damaged or dead cells. Different stem cells have different differentiation potential: the primary stem cells in the embryo can eventually become, after a series of differentiation processes, any of the more than two hundred types of cells in the adult human body as well as extra-embryonic tissue cells, such as the placenta. Other stem cells are limited in their ability to differentiate, and are able to become only a few types of cells or only one type.
A bone marrow transplant is actually a transplant of hematopoietic stem cells, meaning stem cells that have undergone partial differentiation and are now able to develop into blood cells of all types. Such transplantation has been accepted for years as a treatment for many blood diseases, and for other diseases in which it is necessary to restore the blood system and the immune system after medical treatment (see our article "Stem cells in the service of medicine" coming soon).
Today, the researchers aim to grow stem cells in the laboratory in sufficient quantity, which can be used as a source of various types of cells that will be implanted in the body of patients and provide a cure for many other diseases. Finding ways to create suitable cells for a successful and safe transplant is a primary goal of stem cell research these days. In addition to bone marrow transplants, stem cells are also used for a variety of purposes: growing them as a cell culture to test the action of drugs; the study of genetic diseases using stem cells with genetic defects; researching cancers caused by defective stem cells; the study of natural healing processes in the body; and a basic study of the mechanisms of stem cell differentiation and of the development of the embryo from a single egg to a complete organism.
In this article we will deal with the role of stem cells in the body, the history of stem cell research, the research methods used in this fascinating field and the bioethical issues it raises.
from an egg to a human
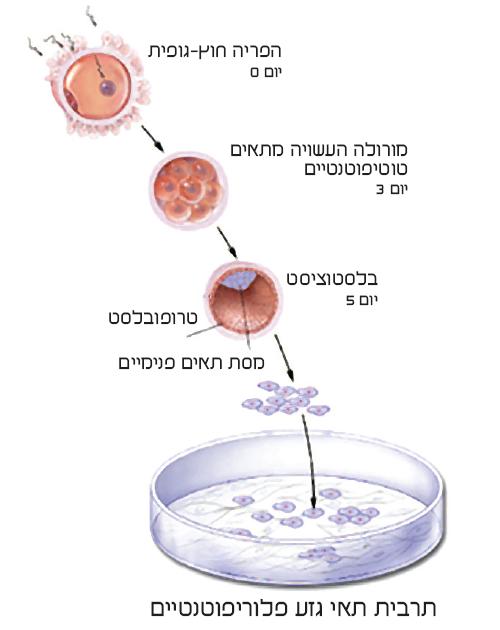
The body's cells originate in a fertilized egg, the zygote, which is the main stem cell from which the fetus will develop during pregnancy. After fertilization, the zygote begins to divide, and within a few days a pool of dozens of cells is formed which still constitute stem cells with the highest differentiation potential.
These cells, called totipotent stem cells, can become during differentiation any cell in the body, as well as extra-embryonic cells, such as the cells of the placenta. The cluster of cells, called the morula, takes the shape of a hollow ball towards the fifth day of pregnancy. The hollow ball, the blastocyst, which at this stage is made of about 100 cells, consists of a shell and a collection of internal cells adjacent to it in a limited area. The mantle layer, the trophoblast, will become the placenta with the rooting of the blastocyst in the uterine lining a few days later, while the cells inside the blastocyst will become the embryo itself. This inner cell mass consists of stem cells with a very high differentiation potential, and they are the source of all body cells in the adult. These cells are called pluripotent stem cells, and they are of great importance in stem cell research.
The first differentiation of the pluripotent stem cells begins at the end of the second week of pregnancy, in a process called gastrulation. Then three layers of cells are formed in the embryo, whose shape at this stage resembles a disc. These are the germ layers that are the source of the various body tissues that develop in the fetus later in pregnancy. The brain, nervous system, sensory organs and skin develop from the outer layer (ectoderm); The skeleton, muscles, connective tissues and the circulatory system develop from the intermediate layer (mesoderm); Whereas the digestive system and the respiratory system develop from the inner layer (endoderm). The stem cells that build the germ layers are multipotent stem cells, meaning stem cells that can differentiate into groups of cells, but none of them can be the source of a complete embryo.
Later, these cells go through several stages of differentiation. Some of them become specific cells in the various tissues, while others continue to reproduce as stem cells even after birth. These cells are the reservoir of somatic stem cells in the body of an adult. When necessary, the somatic stem cells enter the differentiation pathway, at the end of which specific cells are formed. A cell that enters a differentiation pathway, and therefore cannot continue dividing forever, is called a committed progenitor cell and serves as a source for a group of cells of one type or individual types. The hematopoietic stem cells, capable of differentiating into the different types of blood cells and immune system cells, are an example of somatic stem cells that may, when necessary, differentiate into committed progenitor cells and from them into the various blood cells. These cells appear in relatively large quantities in the umbilical cord blood that can be collected at birth, and they are also regularly found in the bone marrow of an adult. Their quantity is relatively large because of the body's need to renew the stock of the various blood cells. In the same way, the amount of stem cells in the intestines is relatively large in an adult. On the other hand, the amount of stem cells that can differentiate into heart muscle cells or nerve cells is low, so damage to these organs may be irreversible.
Somatic stem cells have a relatively low ability to differentiate, and are usually able to differentiate into a small number of cell types. Because of this, their use as cells intended for transplantation is limited to certain diseases only, and is only possible if a sufficient amount of cells can be collected from the donor's body. A bone marrow transplant or a blood transplant rich in stem cells is an accepted medical procedure as a treatment for many diseases related to the blood system. But similar treatments, which may help millions of patients with other diseases that cause irreversible tissue damage, are not possible at this stage, because it is still not possible to obtain a sufficient amount of sorted cells or of somatic stem cells that can differentiate into cells that will replace the damaged cells. Thus, for example, in order to help patients suffering from severe spinal cord injury, cells called oligodendrocytes that produce myelin, the insulating substance that wraps the axons and allows them to conduct nerve signals, must be transplanted. Unfortunately, there is no practical way to receive from another person a donation of oligodendrocytes or progenitor cells capable of differentiating into these cells.
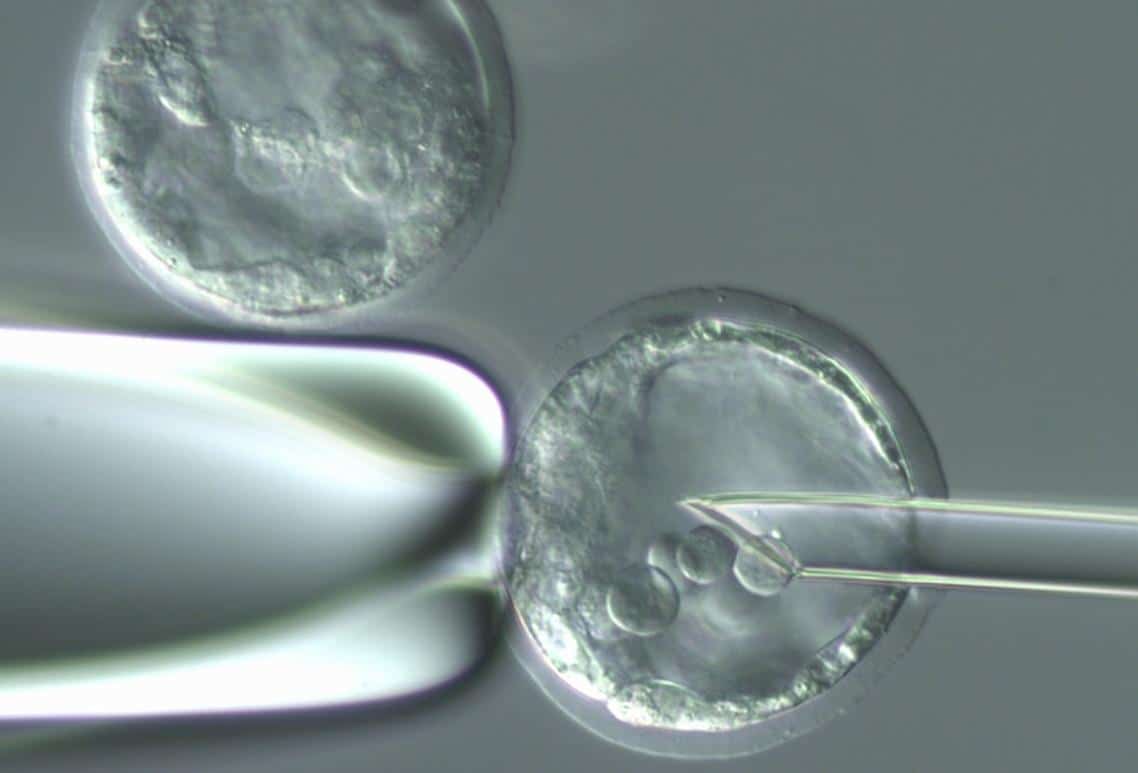
Embryonic stem cells may change the picture. These cells are pluripotent stem cells, which are extracted from the inner cell mass in the blastocyst five days after in vitro fertilization (Figure 1). The embryonic stem cells can, under suitable conditions, continue to multiply as pluripotent stem cells and serve as a source of millions of cells, which under other laboratory conditions will differentiate into the desired cell type. In this way it is possible to obtain a sufficient amount of specific cells intended for transplantation. However, before this medical procedure becomes a hackneyed vision, it is necessary to achieve maximum medical safety. In particular, three main dangers hinder the widespread initiation of clinical trials: graft rejection, infections in the transplanted cells and the development of tumors in the patient's body. These dangers have become clear during stem cell research over the past 150 years.
The ability to differentiate is a double-edged sword
From the 19th century to the present day, stem cell research has progressed in three separate channels - the study of blood system stem cells, the study of tumors in the human body and the development of in vitro fertilization. The first channel is related to understanding the location and function of somatic stem cells in the adult human body. The first to realize that the bone marrow contains cells from which the various blood cells are formed was the German researcher Ernst Neumann, who claimed this as early as 1868. At the beginning of the 20th century, Neumann and others began to use the term "stem cells" also to indicate the hematopoietic cells ("the blood makers ") in the bone marrow, and this is in addition to the meaning that the combination had earlier - cells in the primary embryo. As early as the beginning of the last century, researchers therefore insisted on the sacrifice between the somatic stem cells and the embryonic stem cells.
The first medical application of Neumann's insights was a bone marrow transplant in patients with blood diseases, but the problem of rejection, that is, the body's tendency to reject a transplant containing genetic information different from its own, meant that these transplants only became routine in the second half of the 20th century, when it was developed Drug treatment that prevents rejection. The danger of transplant rejection is expected to be a major problem in the development of other stem cell transplants, and the ultimate solution to this problem may come from unexpected directions.
In the 19th century, researchers from France and Germany became interested in a rare and strange tumor called a teratoma - "monstrous tumor" in Greek. A teratoma is a benign tumor, usually, containing a mixture of cells originating from all germinal layers. Sometimes organ-like tissues are found inside the tumor, and even pieces of skin, whole teeth or hairs. According to the composition of the cells in the teratoma, it is clear that it originates from one or more pluripotent cells, but the manner of its formation is not fully understood. However, one type of teratoma is apparently being deciphered: an ovarian teratoma is thought to originate from an egg that has undergone parthenogenesis, i.e. has begun to divide without fertilization. Other types of teratoma probably develop when the sick person is still developing in his mother's womb, as a result of a defect in the differentiation of embryonic stem cells. This is where one of the dangers associated with transplanting cells derived from the culture of fully or partially differentiated embryonic stem cells becomes clear: if pluripotent stem cells remain in the transplanted culture, they may develop into a tumor. Therefore, ways must be found to get rid of all the pluripotent cells before transplantation, or to cause their programmed death through genetic modification.
Not only the teratoma originates from stem cells, but also other types of cancer. It is hypothesized that many forms of cancer are caused by a series of mutations that occurred in somatic stem cells and the progenitor cells created from them, which began to multiply in an uncontrolled manner. Hence, the study of stem cells in the laboratory, and in particular targeted genetic changes of these cells, can teach us quite a bit about cancer and help in the search for a cure for the various forms of the disease.
Embryos as a source of stem cells
The first attempts to perform in vitro fertilization were conducted at the end of the 19th century, but even here it took a long time before the technique matured and became an accepted procedure, about a hundred years later. The first "test tube baby" was born in Britain in 1978. In retrospect, it turned out that this method, designed to help couples suffering from fertility difficulties, paved the way for the regular production of human embryonic stem cells.
In vitro fertilization involves, in the first stage, hormonal treatment in the woman, which is designed to make several eggs mature at once. In the next step, an average of ten eggs are extracted from her ovaries. These eggs are fertilized with sperm cells, and some of the resulting embryos are transferred to the uterus a few days later. The rest of the embryos are frozen in liquid nitrogen for further experiments. Most of these embryos, whose number today is estimated at hundreds of thousands worldwide, are destroyed several years later if the couple does not wish to conceive again or donate them to another couple.
In some countries, the couple can donate the excess embryos to research. In Israel and many other countries, spouses who do this are not paid, and it is also forbidden to create excess embryos on purpose. Embryos that are found to be defective during microscopic examination, or have a genetic defect detected, are usually destroyed. Genetic defects are detected in a test called a preimplantation test; This is a test that does not harm the developing embryo, and which involves removing one cell, a blastomere, from an eight-cell morula, and transferring it to a genetic test. Among the researchers, there is actually an interest in receiving a donation of damaged embryos, which would otherwise be expected to be destroyed anyway. Stem cells from such embryos are an important tool in understanding the impact of genetic defects on the developing embryo.
The creation of embryos by in vitro fertilization eventually led to a breakthrough in stem cell research. The British researcher Martin Evans (Evans) was familiar with the field of stem cells from his previous studies in teratomas, but he realized that in order to develop more accurate laboratory methods he had to obtain the stem cells from another source. In 1981, Evans, together with his colleague Matthew Kaufman, succeeded in extracting embryonic stem cells from a mouse blastocyst and creating a cell culture from them (Figure 2). A stable stem cell culture from one source is called a stem cell line, and the cells in it can continue to exist in an undifferentiated form as long as the appropriate conditions are maintained. Later, Evans used the stem cell line he produced to create genetically engineered mice. For this work he was awarded the Nobel Prize for Medicine in 2007.
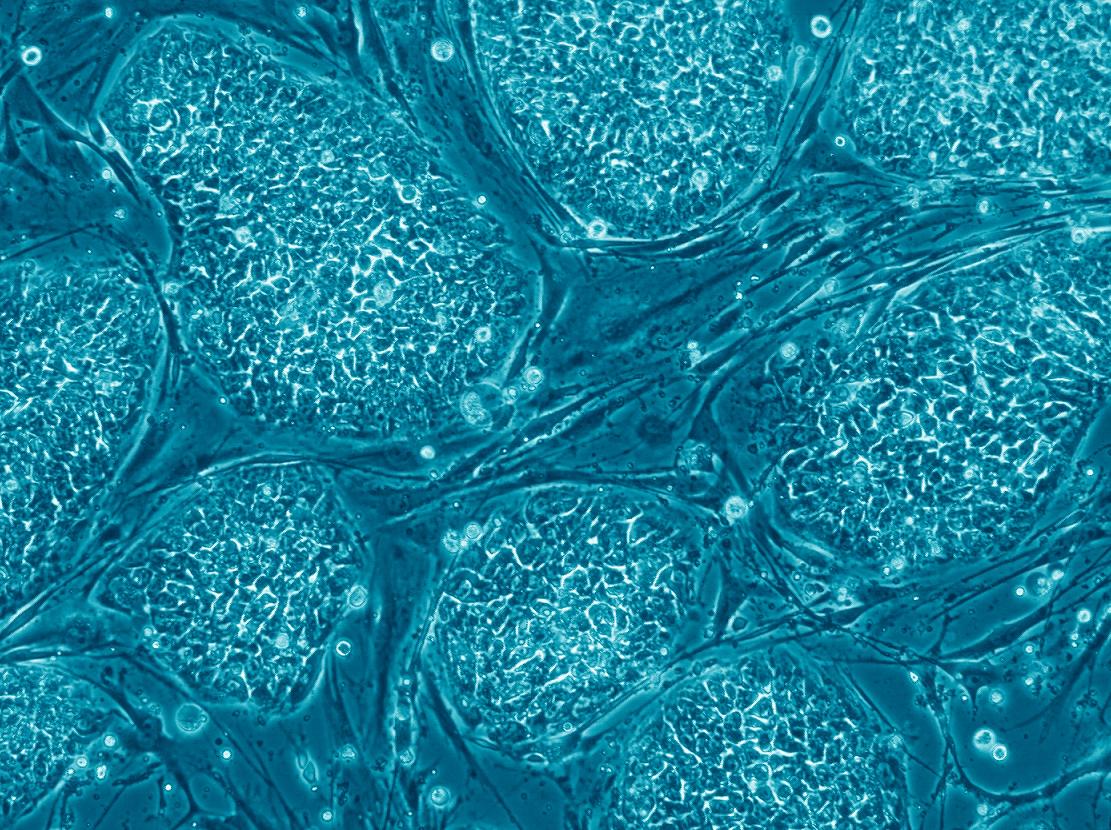
The next breakthrough occurred in 1998. The research group of James Thomson (Thomson) from the University of Wisconsin in the United States, which collaborated with Prof. Yosef Itzkovitz-Eldor of the Technion and the Rambam Hospital in Haifa, was the first to produce human embryonic stem cells and create a line of stem cells from them. The tiny embryonic stem cells are extracted from the inner cell mass in the blastocyst, and for this the trophoblast layer must be punctured and the inner cells removed. In this process the embryo is destroyed and does not continue to develop. The human embryonic stem cells are grown on a substrate of mouse cells, usually fibroblast cells, that have been treated so that they do not divide (Figure 3). It is a sticky substrate that holds the culture and provides it with nutrients and various factors, which the fibroblasts secrete and which are essential for maintaining the properties of the embryonic stem cells.
The mouse cell substrate is problematic for two reasons: antigens may penetrate from the mouse cells into the human cells and cause their rejection during transplantation; And pathogens can move from mouse cells to human cells and cause disease. Because of this, various companies are trying to develop an alternative synthetic substrate before the clinical trials begin, and indeed, the new synthetic substrate developed for the leading biotechnology company Geron will be used to grow cells intended for transplantation.
Recently, the research group of Prof. Benjamin Raubinoff, from Hadassah Hospital, succeeded in growing a culture of human embryonic stem cells in suspension without the cells differentiating. The team of researchers found that when the stem cells were grown in a liquid used for directed differentiation into nerve cells, a significant part of the stem cells did not differentiate. With the optimization of the protocol, a practical way was found to grow in suspension a large amount of human stem cells, which remained pluripotent. The new method has implications for the ability to produce large amounts of stem cells with relatively little effort (Figure 4).
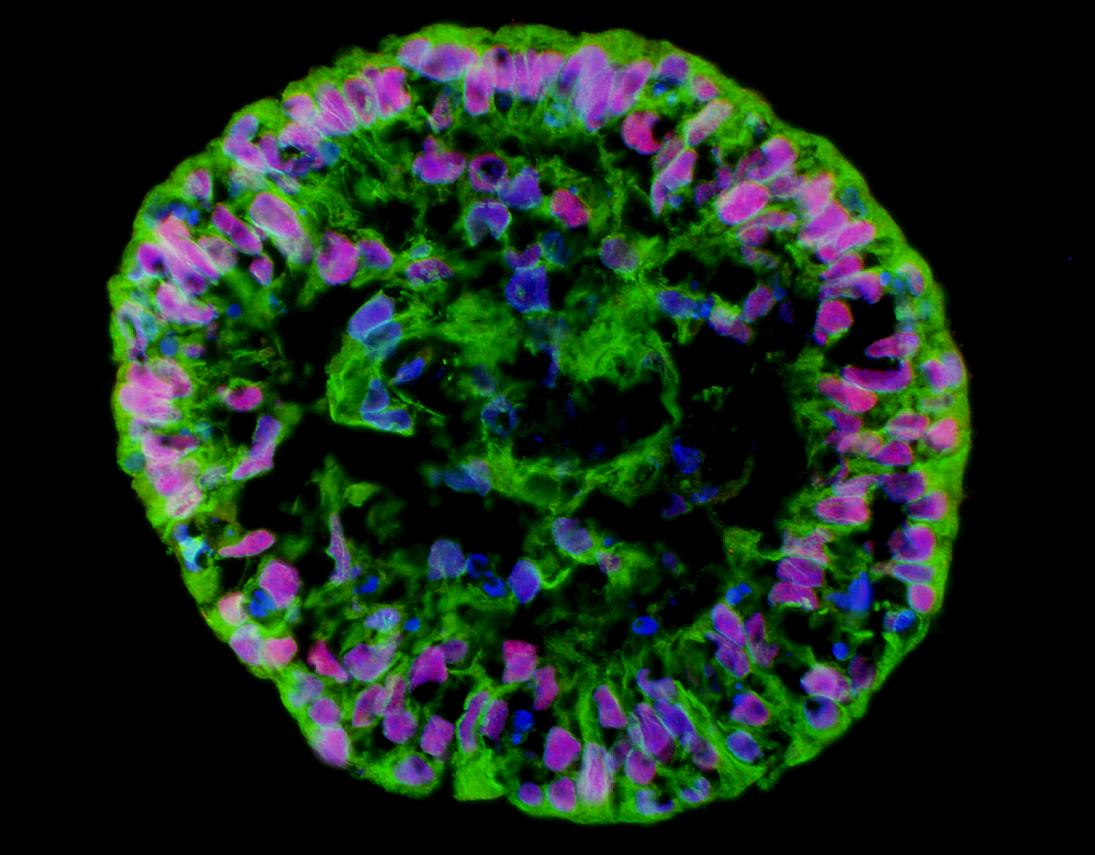
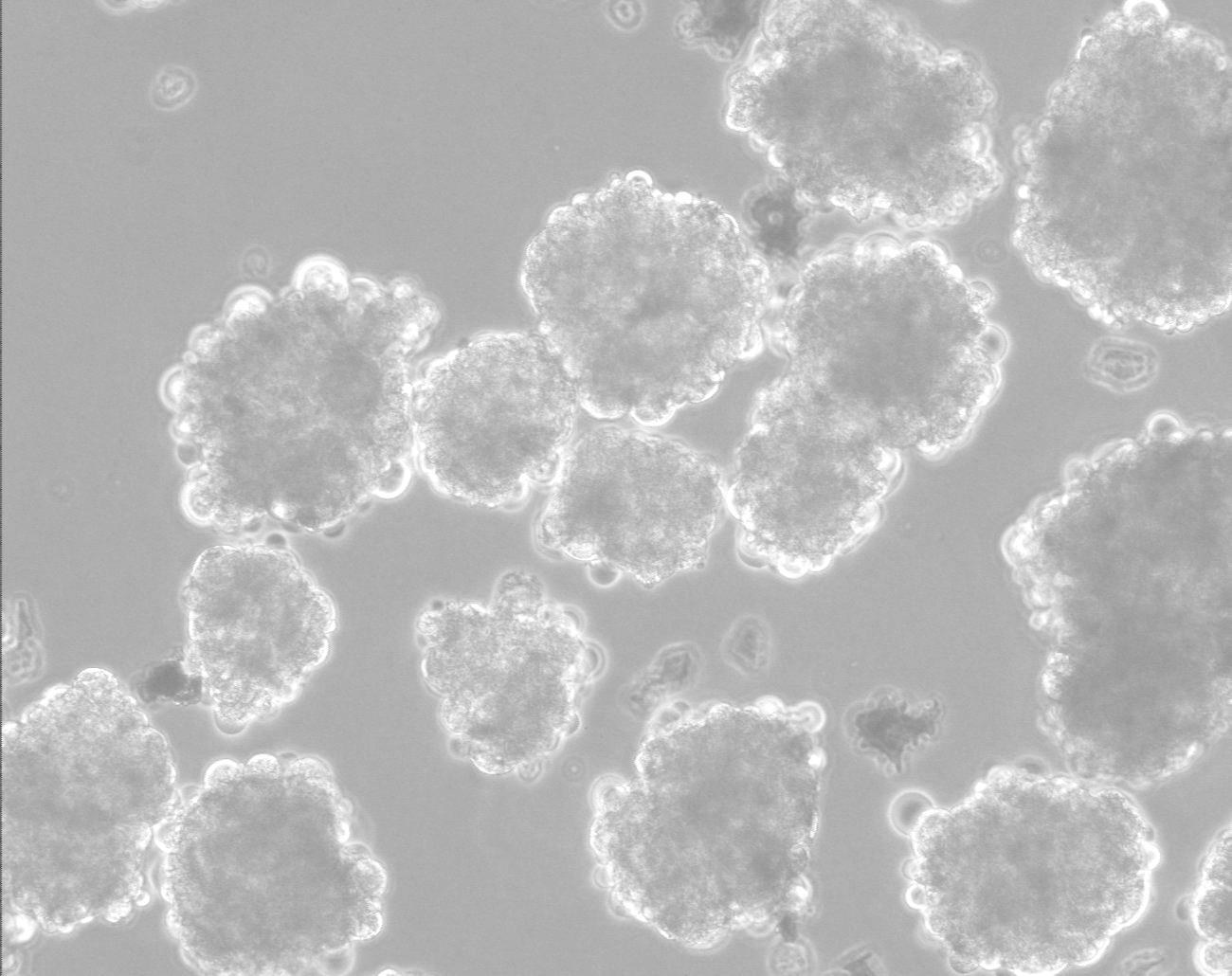
After a pluripotent stem cell culture has grown normally and without differentiating for six months, it can be declared a cell line. Today there are already several hundred such cell lines around the world. Every few months the stem cell culture must be checked again, in order to make sure that it maintains pluripotency. The exam usually includes tracking the rate of division; A microscopic examination designed to make sure that no sorted cells have appeared; Identification of transcription factors that characterize unsorted stem cells, such as Nanog and Oct4; Identification of markers present on the surface of the cells, which characterize unsorted stem cells; karyotype examination with a microscope; and checking for normal growth after freezing, thawing and transferring to a new petri dish. It is also necessary to make sure that the differentiation ability of the cells has not been damaged. For this purpose, a small amount of cells is transferred to a liquid substrate to create a suspension, and it is checked if an embryo-like body (embryoid body) containing sorted stem cells from the three germ layers begins to form (see figure at the beginning of the article). Another way to verify that differentiation has occurred is to inject cells into a mouse with a suppressed immune system (to prevent rejection). Such an injection should create a benign teratoma in his body.
Each cell line provides researchers with an infinite source of stem cells whose properties are known, and from which, if necessary, different sorted cells can be obtained through genetic manipulation or the addition of specific factors. The sorted cells can be used as a culture to test the effect of new drugs before they are put on the market or as a source of cells for transplantation in patients. However, the danger of rejection remains, and in order to overcome it, other methods are needed.
Ethical obstacles
In order to avoid transplant rejection without suppressing the patient's immune system, a potentially dangerous medical procedure, it is required that the genetic information in the transplanted cells be the same as the genetic information in the patient's body cells. The answer to this challenge is medical cloning. Cloning is based on the transfer of a nucleus from a normal somatic cell, usually a skin cell, to an unfertilized egg from which the nucleus has been removed. An electric pulse causes activation, following which the egg begins to divide. When the embryo reaches the blastocyst stage, embryonic stem cells containing the patient's genetic information can be extracted from it. In this way, it is possible to create a personal stem cell line for each patient in need of a transplant, and overcome the risk of rejection.
This method raises objections of two kinds: some argue that cloning human cells, even if it does not result in the birth of a baby, is immoral, and will lead to the legitimization of cloning humans for reproductive purposes; Producing stem cells from an embryo while destroying it is also a bioethical problem in the eyes of many, even if it is embryos that will be destroyed anyway. This issue is related to the question of the moment when the fetus becomes a person. This question is not purely scientific, and in discussing it, cultural and religious considerations must also be taken into account. In the eyes of Catholic Christianity, the moment of conception is the moment of the beginning of life, hence the Vatican's strong opposition to the production of embryonic stem cells. Judaism and Islam, on the other hand, claim that the law of a fetus in the first stages of pregnancy is not the same as the law of a fetus that is about to be born. According to the popular opinion in Judaism, it is possible to use fetal cells for research that will certainly not be transferred to the uterus.
However, the two ethical issues can be dealt with in a different way - through technological-scientific developments that will eliminate the need to harm embryos, and the necessity of cloning to create a personal stem cell line. Two such solutions have indeed been found recently.
Cells repeat in time
The first, simpler solution allows the creation of a line of embryonic stem cells without destroying the embryo. Pre-implantation genetic testing of an embryo created by in vitro fertilization involves removing a single cell - a blastomere - from the morula. If the cell is found to be normal, the embryo can be transferred to the uterus: removing the single cell does not harm the embryo, and it can develop into a healthy baby. Now researchers have succeeded in using this single cell, instead of genetic diagnosis - to create a culture of stem cells.
The second, more complex solution also deals with the rejection problem. The idea behind it is to reverse the direction of differentiation, that is, to take a somatic cell from an adult human body and make it return to the pluripotent state that characterized one of its early ancestors. This is done through a genetic change in the somatic cell, causing it to express certain genes. These genes encode transcription factors, such as the previously mentioned Oct4, that characterize the unsorted stem cell. The resulting cells, called induced pluripotent stem cells (iPS cells), have the characteristics of normal embryonic stem cells: they can divide and remain stem cells or differentiate into cells from the three germ layers. The first success in this field was recorded in the laboratory of the Japanese researcher Shinya Yamanaka, who succeeded in 2006 in returning adult mouse cells to their embryonic state. A year later, Yamanka's group and the group of the American researcher Thomson managed to achieve a similar feat in human cells.
And what's next?
In the coming years, clinical trials on humans are expected to begin, conducted by leading biotechnology companies such as Geron. In the first planned experiment, oligodendrocyte progenitor cells, which will be produced from human embryonic stem cells, will be transplanted into the bodies of patients suffering from severe spinal cord injury. This treatment may help patients move their limbs. Nerve cells and other cells related to them, which will be produced from stem cells, will be used in a variety of innovative treatments for diseases affecting the brain and nervous system, such as Alzheimer's disease, Parkinson's disease and Lou Gehrig's disease (ALS). If regenerative medicine, which relies on transplanting cells differentiated from pluripotent stem cells, succeeds in treating these diseases and other serious diseases, such as heart disease, millions of patients around the world will have new hope.
A fascinating topic, which stem cell researchers are still far from understanding, is the collection of epigenetic mechanisms - that is, the hereditary mechanisms that do not derive from the DNA sequence - which cause the differentiation of stem cells and the development of organs in the growing embryo. It is known that differentiation is characterized by limiting the expression of certain genes, and that the factors that activate it are chemicals secreted by other cells, physical contact with neighboring cells and molecules in the fluid around the cells (microenvironment). However, the detailed picture has not yet been revealed. Perhaps when these details become clear, we will be able to fulfill the greatest promise of stem cells - the creation of whole organs intended for transplantation.
Frame 1: And what about Israel?
Do you also research induced pluripotent stem cells (iPS cells)?
Generating induced pluripotent stem cells is an important breakthrough. We joined research in the field, and published three articles in it in the last year and a half. In the last article we compared a genetic disease model in embryonic stem cells and a similar model in iPS cells. We discovered that in many respects the models are similar, but at a certain critical point they differ. Despite the similarities between the two cell types, it is important to continue to explore the differences.
Will these cells replace the embryonic stem cells or will their medical use be limited?
The researchers are on the verge of clinical trials with embryonic stem cells in the United States. On the other hand, the medical use of induced stem cells is still not safe, because genetic manipulation is currently necessary to create them. Therefore I can say with certainty that clinical trials in embryonic stem cells will precede clinical trials in induced stem cells. On the other hand, the induced cells bring with them a huge innovation, which is the possibility of producing the induced cells from the patient's own body and returning to him cells that are genetically identical to his own body cells. In this way, rejection of the graft will not occur.
Where does the research stand in terms of the ability to control the differentiation of the stem cells?
In the last ten years, many protocols have been developed to differentiate embryonic stem cells into different cell types, such as skin, nerve and liver. The main difficulty at the moment is to get a culture containing only sorted cells. There are methods that allow us to obtain an almost clean culture of sorted cells, for example - with the help of a cell scanner. However, the amount of cells obtained by these methods is relatively small.
In the clinical trials planned in the United States, in which cells will be transplanted into the bodies of patients whose spines have been seriously injured, the intention is not to use a cell scanner, but rather to use cultures that are not completely clean in terms of cell composition, despite the obvious danger of developing tumors from unsorted cells. One of the proposals is to transplant the cells at the stage where they are progenitor cells, so that they undergo the last differentiation in the patient's body. In this way, the danger of tumor development will be reduced, and another advantage will be achieved - the transplanted cells will regenerate the patient's tissue over time. The danger is that they may not differentiate correctly, and in order to find out this point it is necessary to continue the basic research.
How can you deal with the danger of tumors?
Transplantation of unsorted stem cells is dangerous. We have shown for the first time that these tumors are benign, yet this is a serious problem. We have developed some strategies to overcome it. First, we developed a method to separate the types of cells, so that we can transplant only cells that do not cause tumors. Second, we showed how the tumor can be eliminated if it is formed, or alternatively use genetic manipulations that include the introduction of a "suicide gene" that will cause the planned death of the cells that may form a tumor.
Where is Israel placed in regards to results and achievements in stem cell research?
In terms of the absolute number of studies, Israel ranks second-third in the world, next to Great Britain, and in a comparative study it was found that researchers from the country were involved in half of the 20 important articles in the field. This is despite the fact that government support for stem cell research is very limited.
Final question: Do you think it will be possible to succeed, one day, in growing whole organs from stem cells intended for transplantation?
This is a very important question. Embryo formation involves two processes: differentiation and tissue formation. We succeed in bringing about differentiation in the laboratory, but still fail to create a complete tissue. Work in this direction is also being done in my lab, and we have some new ideas. Creating organs in the laboratory is one of the major long-term goals of stem cell research.
Frame 2: The word of the legislator
Due to the bioethical issues raised by the production of stem cells from embryos and the use of cloning techniques, the issue was required by the legislatures of many countries. Each country chose its own way, according to the "scientific climate" in the government circles and the public, the involvement of the public and extra-parliamentary organizations in ethical questions and religious and cultural considerations. A review of the legislation in the various countries reveals a fairly broad agreement on two issues: a ban on cloning humans for reproduction and a ban on the use of human embryos over two weeks old for research purposes.
Embryonic stem cells and the church
The legal restrictions in the various countries can be divided into three levels. The strictest countries forbid any stem cell research. This group mainly includes countries characterized by a strong influence of the Catholic Church, such as Poland and Ireland. The second group includes countries that allow the production of embryonic stem cells, but do not allow the creation of pre-designated embryos for this purpose. Stem cells can therefore only be obtained from embryos created for the purpose of reproduction in vitro fertilization, and which are no longer needed for this purpose. This group includes Israel and Canada. The most liberal policy allows the creation of embryos for research purposes, but limits their growth and use to the initial period of their development. Great Britain and China belong to the third group.
Between Bush and Obama
Not every country fits into one of these three groups. Germany allows research in embryonic stem cells, but prohibits their production in its territory. The situation in the United States is even more complex. There is no federal law prohibiting embryonic stem cell research, and each of the states of the United States has the right to determine for itself what is allowed and what is not allowed in its field. The scope of the restrictions in the different countries is diverse, starting with a complete ban and ending with a permissive and liberal policy. In permissive countries, there is usually massive private investment in stem cell research, and the state itself is allowed to invest generously in research - but federal research funds are subject to the president's decision. George W. Bush, when he served as president, opposed embryonic stem cell research, but allowed little federal funding for stem cell lines created before August 2001. Obama took the opposite position, reversing Bush's restrictions on the research of hundreds of stem cell lines created over the decade the last one. However, the federal funding is now conditional on the approval of the ranks in accordance with ethical guidelines formulated last year, and it is not at all certain that all the ranks, even those created before Bush's declaration, will receive the generous funding of the Obama administration.
Box 3: A question of epigenetics
Epigenetics is a field that deals with the study of hereditary mechanisms, which are involved in the determination of the phenotype and gene expression and which do not derive from the DNA sequence. The pluripotency feature of embryonic stem cells is primarily determined by a small number of transcription factors. Among these transcription factors we can mention Oct4, the Sox2 nanogut, which are expressed in human embryonic stem cells. The experiments in which iPS cells were first produced showed that the introduction of a small amount of genes encoding transcription factors such as these is able to cause a sorted somatic cell to return to a pluripotent state.
The transcription factors in the stem cells influence the expression of genes that code for proteins, which determine the epigenetic properties of the cell. Thus, for example, these proteins may cause methylation of DNA, that is, the addition of a methyl group (CH3-) to a specific place in DNA, which causes the cessation of the expression of some of the transcription factors that characterize the pluripotent state. This methylation, which will also be inherited by the cell's descendants (de novo methylation), affects exit from the pluripotent state and the beginning of differentiation. On the other hand, demethylation of specific places in histones - the proteins involved in the packaging of DNA in chromatin - may cause the transcription factors that characterize the pluripotent state to continue to be expressed, thus keeping the stem cells in their pluripotent state.
In addition to the methylation and demethylation mechanisms, there are probably many other epigenetic mechanisms that researchers have begun to discover in recent years. These mechanisms include post-translational modifications in histones, structural changes in chromatin and changes in the architecture of the nucleus. A more complete understanding of the epigenetic mechanisms and the complex relationships between them will enable an understanding of the processes of regeneration and differentiation in different stem cells, and will allow us to more effectively control the stem cells growing in culture and the stem cells in the human body.
About the authors
Judy Melamed-Katz is a chemist completing doctoral studies in microbiology at the Hebrew University; Worked at Teva and MZP, and currently engages in writing and scientific education.
Aryeh Melamed-Katz is an electronics engineer and doctor of physics, a graduate of the Weizmann Institute of Science; Currently engaged in writing, developing science education programs, lecturing on science topics and providing scientific consulting services.
Aryeh's blog
ariejudy@gmail.com
for further reading:
* NIH's website on stem cells, with an up-to-date list of sources and the new guidelines for the approval of stem cell lines:
http://stemcells.nih.gov/
* Animation about stem cells:
* Stanford Encyclopedia of Philosophy article on the ethics of stem cell research:
http://plato.stanford.edu/entries/stem-cells/
* The report of the Bioethics Advisory Committee of the Israel National Academy of Sciences on the use of embryonic stem cells for medical research purposes:
http://bioethics.academy.ac.il/hebrew/report1/Report1-h.html
* A collection of articles on stem cells in Nature:
http://www.nature.com/stemcells/index.html
* Key articles in the history of stem cell research:
A. First creation of a mouse embryonic stem cell line:
Evans, MJ and Kaufman, MH, "Establishment in Culture of Pluripotential Cells from Mouse Embryos", Nature 292(5819):154-156 (1981).
B. First creation of a human embryonic stem cell line:
Thomson, JA, Itskovitz-Eldor, J., Shapiro, SS, Waknitz, MA, Swiergiel, JJ, Marshall, VS and Jones, JM, “Embryonic Stem Cell Lines Derived from Human Blastocysts”, Science 282(5391):1145- 1147 (1998).
third. First creation of mouse iPS cells:
Takahashi, K. and Yamanaka, S., "Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors", Cell 126(4):663-676 (2006).
d. Generating iPS cells from a human:
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K. and Yamanaka, S., "Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors", Cell 13(5):861-872 (2007).
Yu, J., Vodyanik, MA, Smuga-Otto, K., Antosiewicz-Bourget, J., Frane, JL, Tian, S., Nie, J., Jonsdottir, GA, Ruotti, V., Stewart, R. , Slukvin, II and Thomson, JA, "Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells", Science 318(5858):1917-1920 (2007).
God. First medical cloning of a human embryo:
French, AJ, Adams, CA, Anderson, LS, Kitchen, JR, Hughes, MR and Wood, SH, “Development of Human Cloned Blastocysts Following Somatic Cell Nuclear Transfer with Adult Fibroblasts”, Stem Cells 26(2):485-493 (2008).
and. First use of a single blastomere as a source of a human embryonic stem cell line:
Klimanskaya, I., Chung, Y., Becker, S., Lu, SJ and Lanza, R., “Human Embryonic Stem Cell Lines Derived from Single Blastomeres”, Nature 444(7118):481–485 (2006).

3 תגובות
The article fascinated me. This important topic is presented here in such a bright and exciting way. Thank you.
Excellent basic article, thanks.
Regarding the clinical trials with embryonic stem cells - it is very possible that in addition to the Geron company's trial with spinal cord injuries, a trial for the treatment of Stargardt's visual atrophy will soon begin in the US.
Advanced Cell Technology expects to receive the FDA's answer regarding the approval of the trial towards the end of August, and if it is approved - it will actually start three months later.
The success of this trial will also pave the way for trials in the treatment of AMD.