Searching for the biological role of peptides, led to the discovery of a new branch of possibilities in the field of medicine
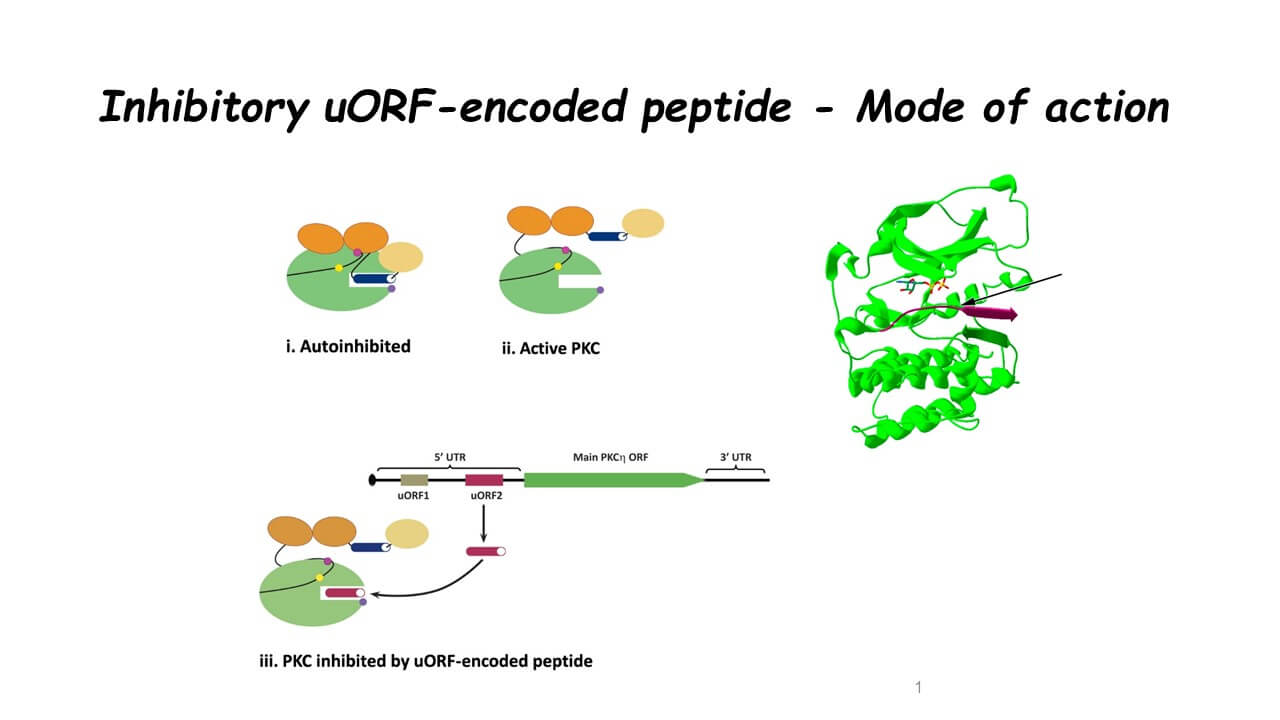
Peptides are short molecular chains of amino acid. In a certain sense, they can be seen as a kind of short proteins, which are also built from chains of amino acids. Proteins are the main "machines" that activate the life processes in the cell, but their short "brothers", the peptides, play relatively few roles. For example, they can stop or slow down the process of translating the genetic information by which proteins are built in living cells. In this way, they can be compared to deceleration lanes on a highway.
But - as Frank Sinatra once sang, is that all? Don't peptides have their own biological role? Prof. Ata Livna from the Faculty of Health Sciences at Ben-Gurion University of the Negev, along with her research group, tried to answer this question.
"This is a long-term study," says Prof. Livna, "it is very difficult to locate small peptides, but on the other hand, the significance of the success may lead to the development of a new source of biological drugs." In the study, which was carried out with the help of a research grant from the National Science Foundation, the members of Prof. Livna's research group were able to isolate a short peptide and show that it inhibits a network of proteins (enzymes) responsible for adding phosphorus atoms to certain proteins - thereby changing their activity.
Adding phosphorus atoms to molecules (for example, protein molecules), stimulates many processes in cells, and among other things, it is an important step in the processes of transmitting signals and messages in cells. These signaling pathways control key processes in cells, including cell division, cell death and especially cell growth and proliferation, a process that leads to the development of cancer.
In this study, Prof. Livna and her team were able to point to the existence of a new control system in cells, based on natural peptides originating from a region of the genetic sequence, which does not code for the main protein. The researchers demonstrated the action of the peptide as an inhibitor of cancer cell survival, tumor progression and even the spread of metastases.
This ability of the peptide to act as an inhibitor stems from the genetic sequence that encodes it, which contains a series of amino acids that appears as a sort of "fingerprint" found in all protein kinase C enzymes. Surprisingly, this "fingerprint" was found on the short peptide, which indicated that it can be used as a specific inhibitor of the phosphorylation activity in the protein network - thus inhibiting its activity.
Proteins (and especially proteins from the protein kinase group, which has more than 500 different proteins) convey vital information inside the cells, and more than once, they dictate the fate of the cell. A substance that inhibits the activity of these proteins also blocks the chain of instructions they transmit inside the cell. In this way, if the communication chain of the proteins signals to a cancer cell to continue dividing and multiplying, because then the inhibition of the messages that pass through this chain, will slow down the spread of the cancer cells.
In studies carried out in mice, the researchers were able to show that the short peptide can inhibit the development of breast cancer, as well as the development of cancer metastases in the lungs. The mechanism of action of the peptide is based, among other things, on the fact that it inhibits the genetic damage repair system in the cancer cell, which more than once causes the death of the cancer cell. Indeed, the study showed that treatment of breast cancer cells using the peptide - sometimes in combination with chemotherapy - may cause the cancer cells to die.
"Some of the biological drugs accepted today in the treatment of cancer are based on inhibiting the activity of protein kinase molecules," says Prof. Livna. "We believe that a clear demonstration of the role of the peptide we found is only the tip of the iceberg. Now that we know that at least some of these peptides have a biological role, we can begin to discover the role of many more, and use them in the development of advanced medical treatments."
Also participating in the study were Prof. Esti Yager-Lotam, and Prof. Moshe Alkabats, as well as Divya Ram Jayaram, Sigal Frost, Hanan Argov, Vijayasteltar Balsma Lijo, Nikhil Ponwar Anto, Amita Muraldaran, Assaf Ben Ari, Roz Sinai, Ilan Smoli, Ofra Novoplansky, Noah Iskov, Dr. Debra Tuiver and Han Kiser.
Life itself:

Prof. Ata Livna, mother of two graduates, has been practicing yoga for years, regularly visits the gym and especially likes walks on the beach. Lover of culture, art and spending time with friends. "When I had to choose my 'life profession,'" she says, "I debated a lot between two loves: science and architecture. I finally chose science. So if I wasn't a scientist I would probably be an architect.
An interesting coincidence that accompanied her current research, led to the fact that the gene that encodes the information for the structure of the studied peptide, was marked with the Greek letter Eta, as the first name of Prof. Livna.
More of the topic in Hayadan:
- Technion researchers propose the launch of a new international project in the field of immunology
- Synthetic substitutes for antibodies
- Viruses communicate with each other - and that's how they decide whether to multiply or go into a dormant state
- The mechanism that may cause diabetics to develop Parkinson's has been deciphered
- Israeli researchers deciphered the mechanism of action of the "predatory bacterium"
