The researchers use CRISPR. To insert into the Y chromosome of the mosquitoes a genetic segment that causes a mutation in the X chromosome next to it, during the creation of the sperm cells. As a result, only male mosquitoes hatch
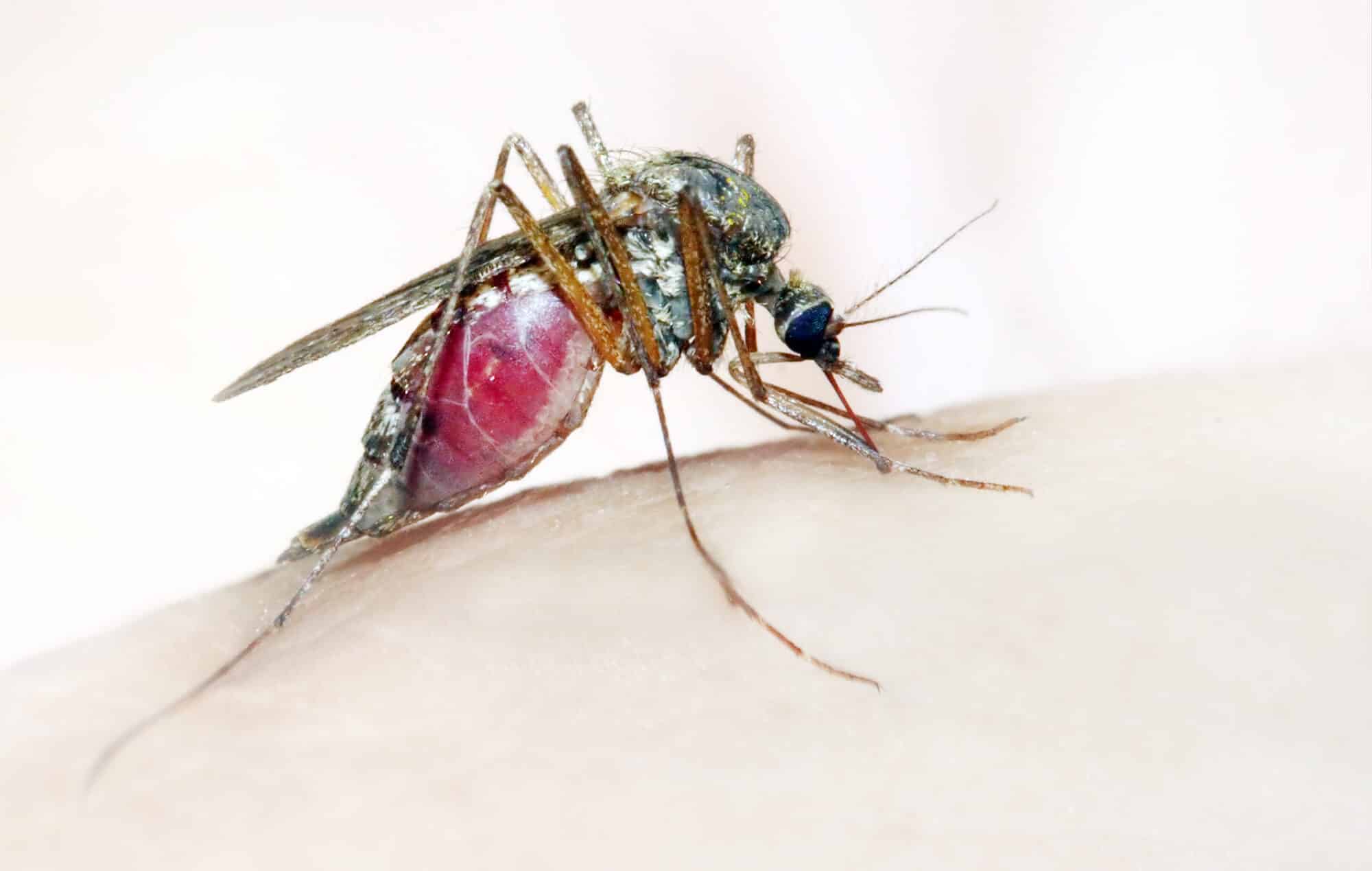
Malaria or swamp fever is still rampant today, mainly in hot and poor countries in Africa and Southeast Asia. The disease in which about 200 million people are infected every year and about 400 thousand of them die, most of them under the age of five. In the laboratory of Dr. Papthanos Philippos from the Department of Entomology in the Faculty of Agriculture of the Hebrew University, CRISPR technology is used to edit the genome of the Anopheles mosquito populations and thus eradicate mosquito populations that transmit the disease.
What is the question?
How can the mosquitoes that transmit the malaria parasites be harmed, by means of a mutation in the genome of the male mosquitoes?
Malaria is caused by a parasite known as Plasmodium, whose life cycle includes the infection of several Anopheles species of mosquitoes, the most common of which is Anopheles gambiae the cause of morbidity in East Africa. The female mosquito feeds on the blood of the host it bites, and in the process transfers the single-celled parasites into the human bloodstream, where they damage red blood cells and cause anemia. The males, by the way, do not feed on blood and therefore do not pose an epidemiological danger. The solution must therefore include an intervention in the life cycle of the parasite.
There is still no vaccine for malaria, and even the existing drugs that are given to travelers to malaria-affected areas have side effects. Moreover, the mosquitoes carrying the parasite develop resistance to the existing drugs. Using DDT for pest control purposes, which was common in the 40's, is illegal today due to the fact that apart from mosquito larvae, it destroys every animal and plant in the area.
In the last 20 years, another solution has emerged: the release into nature of sterile males who will mate with the females and actually cause a significant reduction in the next generation. This is an expensive method, because the release has to be repeated from time to time, in order to compete with the fertile male mosquitoes that exist in the wild. In addition, 100 times more mosquitoes need to be released than the mosquitoes that are naturally found in the same habitat. And this, in addition to mosquitoes from nearby swamps that can penetrate the habitat.
The insect genetics laboratory led by Dr. Papthanos Philippos, a senior lecturer in insect biological engineering at the Faculty of Agriculture, Food and Environment of the Hebrew University in Rehovot, has an innovative solution for which he was even awarded funding from the National Science Foundation, the body that encourages basic science in Israel.
To compete with the fertile male mosquitoes that exist in the wild, you have to release 100 times more mosquitoes than the mosquitoes naturally found in the same habitat. And this, in addition to mosquitoes from nearby swamps that can penetrate the habitat.
The researchers use genetic editing tools based on CRISPR technology. Using this method, a genetic segment is inserted into the Y chromosome of the mosquitoes that causes a mutation in the X chromosome next to it, during the creation of sperm cells (spermatogenesis). This action does not harm the male offspring, but causes the death of the females, who inherit the mutagenic X chromosome from their father. Release of mosquitoes Males that have undergone such genetic editing are expected to suppress the mosquito population in the release area until local extinction, without causing the extinction of the entire species.

The development of the genetic editing method CRISPR earned the researchers Emmanuel Charpentier and Jennifer Daunda the Nobel Prize in Medicine and Physiology for 2020. The idea of inserting genes into the exact place where you want them to have an effect in a plant or animal has already begun to change the face of medicine and agriculture.
"Our research focuses on developing ways to suppress and even eradicate mosquito populations using methods that are safe for the environment," says Dr. Philippos. "The number of fertile females is the factor that determines the size of the population and in the case of Anopheles they are the ones that cause the enormous health and economic damage. At the same time, we are developing another technology in which we release into the wild male mosquitoes that carry a genetic sequence that is expected to spread over time in the population and will not require the constant release of mosquitoes. This is called Gene Drive.
In fact, Dr. Philippos, his students and research partners use the main way that defines the term evolution: the process of genetic change in a population of organisms over generations through changes in the frequency of appearance of alleles in the population.

Dr. Philippos says that this research is still in the laboratory stage. "In the first step, we develop sterile males using the same method so that we can compare the method with the methods used by the other laboratories dealing with this around the world. But it's worth remembering that no organism engineered using CRISPR has yet been released into nature." He adds that it will take a long time until we reach the full application stage. At the same time, it will be necessary to settle the regulation on the field of genetic engineering, to ensure that transgenic varieties do not take over the environment.

Life itself:
Dr. Philippos, Greek by origin, studied at Imperal College in London. He did his post-doctorate at Caltech, California, from where he moved to the University of Perugia in Italy, until finally in 2018 he moved to Israel, following his Israeli wife, also a scientist whom he met when they were both in post-doctorate at Caltech. "There is a Greek saying that if you see a boat on top of a mountain, it means that a woman placed it there." Women can do anything. This is the answer he gives to those who wonder what a Greek researcher does in Israel.
According to him, he specifically chose the insect genetics laboratory at the Faculty of Agriculture because he was fascinated by the future applied implications for the benefit of humanity. At the same time, he investigates uses of the same technology also against insects harmful to agriculture.
For the article on the Voice of Science website
More of the topic in Hayadan:
- Mosquito on the head
- agriculture, malaria and other troubles
- An accurate assessment method of genome editing with CRISPR
- Genetic engineering and animal-human hybrids: How China is leading a global divide in controversial research
- The failure of the gene-edited babies experiment in China proves that we are not ready for gene-editing of human embryos
Tags:
