In discussing a report in the science committee, the chairman of the council stated that this is the number 1 cause of infections in hospitals, and that experiments with these bacteria are "very problematic" and must be conducted under supervision. In addition, the council raised its concern about the uncontrolled use of toxins such as Botox * The chairman of the committee, MK Uri Makleb, called on the council to demand proper cyber protection among the entities that deal with dangerous substances: "This is one of the most sensitive issues today, certainly when we are talking about disease-causing agents that can harm many of the population."
On Tuesday, the Science and Technology Committee held a discussion on the subject of the 'Report of the Council for the Regulation of Research in Biological Pathogens on its activities, for the years 2015-2016'.
The chairman of the committee, MK Uri Makleb: "We are holding our regular reporting discussion, at the same time we would like to dwell on two issues - the duration of the approval procedure for adding or removing generators, is it possible to improve this process that we talked about in the past, which is a process Relatively long. Another issue is the supervision of the various entities that use generators, we know that effective supervision leads to deterrence and compliance with the procedures."
Professor Bracha Reger, chairman of the (outgoing) Council for the Regulation of Research on Biological Pathogens: "We are the only country that has a law in this field, in the US there are all kinds of laws that cover and do not cover the issue."
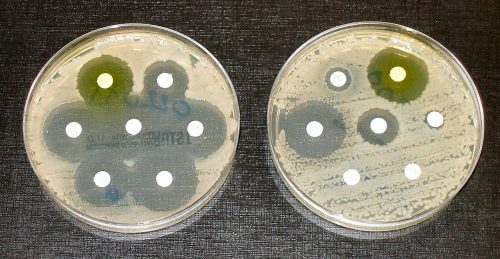
To MK Makleb's question, "Is the law too strict, does it cause regulation that prevents us from activities, makes it difficult for the laboratories?", Reger answered: "My colleagues in the US think that the situation here is quite well organized, I even received requests to give lectures on what we are doing. At the same time there is the issue of changing and removing the generators is quite problematic as it sometimes takes time. Sometimes viruses or bacteria that we don't know break out quickly, such as Zika. We now have a problem with the development of antibiotic resistant bacteria, this is the number 1 problem in hospitals causing infections. I assume that many researchers will want to get into the matter and we would also like to include these bacteria in the list that requires approval for research within the framework of the law, it must be done quickly as this is very problematic research."
Attorney Moriah Ben Tzur, Legal Bureau, Ministry of Health said: "When talking about changing the list, it was determined that it should be by changing regulations, as has already been done here in the past. When the council decides that it wants a change, it brings the wording, which is subsidiary legislation, before the minister. We have to go to the Ministry of Justice with the wording and then bring it before the committee, it takes some time, usually a few months."
Later, Reger noted that there is also complexity with various toxins included in the list: "Botox, for example. Doctors and even beauticians use it, it is a significant toxin but they use it freely. Often and in small doses it is also used in neurological diseases so it is impossible to stop, we are trying to promote supervision but the problem is the quantities. In the American law, the prohibition is defined above a certain weight, this should be fixed here as well, there are medical studies that only require a small amount and they should be exempted. The problem is that in the field of cosmetics there are those who use fake Botox and there was a case in Haifa that caused a lot of damage."
Dr. Ronit Miyhos, Scientific Advisor at the Council for the Regulation of Research on Biological Pathogens: "The problem is that if there is no obligation to report small amounts, I will not be able to know who ordered which amounts. For me, the person in charge of safety at the institution is responsible for the amount after reporting to us."
Dr. Leah Valinsky, Director of the Molecular Biology Unit, Ministry of Health: "We need to check if there are institutional committees that make things more difficult and easier. In my humble opinion, the very fact that there is a law and people have to report and we know what is in every refrigerator is an achievement. What they don't know is put in a bag and sterilized. Regarding Botox, I personally would not inject myself with this toxin."
MK Makleb: "We asked you in the past to examine the cyber issue, where do things stand?"
Rieger: "We looked into the matter with the Cyber Authority, they did not find any problems with us at the moment. They instructed us that if there is a fear of information leakage at one of the centers we will contact specific advice. Most institutions have protective measures, but we keep our eyes open."
Dr. Yonat Shemer, the (incoming) chairman of the Council for the Regulation of Research on Biological Pathogens: "We plan to consult and establish criteria for the issue of information security of high-level pathogens and ask all the institutions and companies that conduct experiments on the substances on the list to comply with the requirements."
Dalia Zager, Head of the Safety Unit, Weizmann Institute of Science: "We know what everyone is buying, this year we added that every scientist should have a safety plan - an annual work plan, we developed software in which all the risks are listed, and every scientist has to write down everything he has as part of it" .
MK Makleb: "We ask that the regulation of the cyber field be done under the supervision and accompaniment of the Authority, they be consulted again, this issue is one of the central threats today, certainly when it comes to generators whose spread could lead to extensive damage to the population. The schedule is also very important, it must be promoted quickly. Along with this, we call to re-examine the shortening of the time frame for trial approvals by the various parties, so that this does not lead to a delay in research and development."
See more on the subject on the science website:
