The Golgi system, a small but crucial organelle in the cell, has its own "breakdown" mechanism
A tendency to break under pressure is not a desirable feature, but when it comes to the cellular organelle known as the Golgi system - this is part of its work plan. Weizmann Institute of Science scientists came to this conclusion after discovering a surprising cluster of proteosomes - molecular machines for breaking down proteins inside cells - around the outer membranes of the Golgi organelle. The research findings, recently published in the scientific journal Nature Communications, indicate that the organ tends to break down in response to stress as part of the control mechanism for its activity. This discovery may pave the way for new treatments for cancer and other diseases characterized by defects in the Golgi system.
About 30% of the proteins in the cell pass through the Golgi system, so this small organelle has quite an influence on what happens in our body. In fact, it serves as a sort of "passage station" for most of the proteins in the cell on their way out - to the cell membrane or out of it. It can be said that the Golgi system is designed similarly to an IKEA store: the proteins that enter it must pass through all the internal sections; Along the way they collect tags - such as sugar molecules and other molecules - and at the end of the last corridor they are assigned "delivery vehicles" that drive them to their destination. Dr. Ron Ben-Yair from Dr. Yifat Marble's laboratory in the Department of Immunology surprisingly discovered that these organelles also have their own "dedicated" pool of proteasomes. "We wondered what protein-degrading machines were actually doing there," says Dr. Avital Eisenberg-Lerner, a senior faculty scientist in Dr. Marble's laboratory, who led the research alongside Dr. Ben-Yair and Noa Hezekiah.
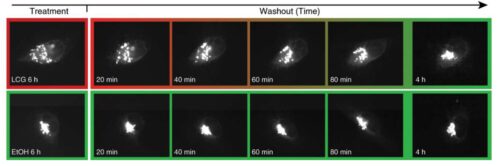
It is known that the Golgi system breaks down when the cell divides or dies, but it was not known if this process is actively controlled in the cells - and if so, how. Following the exposure of the proteasome reservoir, the researchers hypothesized that stressful situations may lead to the disintegration of the system in a controlled manner. To test their hypothesis, they created stress situations of two types: loading the system with proteins, which created "traffic jams", and disrupting the addition of sugar molecules to proteins that passed through the system. According to the hypotheses, the system did not withstand the pressures - and fell apart. The researchers saw that due to the resulting pressure, the GM130 protein - the glue that holds the organ - actually broke down.

The activity of the Golgi system varies greatly from one cell type to another. To examine the activity of the system in a disease state, the researchers chose multiple myeloma cells - cancerous plasma cells found in the bone marrow, since in these cells the load on the Golgi is high due to increased production of proteins (antibodies) and their secretion.
The researchers saw that while in healthy cells, the Golgi system reassembles itself shortly after its disintegration, in multiple myeloma cells when they caused the Golgi organelle to disintegrate - a process of programmed cell death began. However, when the researchers blocked the activity of the proteasomes, the Golgi system remained intact - even when the pressure was too much to bear. In addition, the addition of the GM130 protein to the Golgi also moderated cell death - even under extreme stress conditions. In other words, the degradation of GM130 proteins by the proteasomes is indeed the cause of the collapse of the Golgi system and cell death. These findings open the door to multiple myeloma treatment based on attacking the Golgi system of the cancer cells.
To test this idea, the researchers treated a model of multiple myeloma in mice using a molecule that creates congestion in the Golgi. They discovered that within a week the molecule significantly reduced the number of cancer cells in the bone marrow and spleen of the mice. "Because the Golgi system is overactive in multiple myeloma, the drug worked in a highly targeted manner - while eliminating the cancerous cells, while the healthy cells easily recovered from the breakdown of the organelle," says Dr. Eisenberg-Lerner.
"It is possible that the Golgi organelle disassembly mechanism is a kind of cellular sensor," says Dr. Marble. "The organ quickly breaks down in stressful situations and reassembles itself when the stress is relieved; But if the condition persists or worsens, the disruption in function and breakdown results in the activation of programmed cell death. These findings have implications for understanding the activity of proteasomes in cells and possible treatments not only for several types of cancer, but also for many other diseases. For example, in the most common degenerative diseases of the brain, Golgi activity goes wrong. If we find a way to control it, we may also be able to control the rate of progression of these and other diseases."
The research was done in collaboration with the laboratories of Dr. Uri Avinaam, from the Department of Biomolecular Sciences, and Prof. Idit Shahar, from the Department of Immunology, at the institute.
About 30% of the proteins in the cell pass through the Golgi organelle, which serves as a sort of "passage station" for most of the proteins in the cell on their way out.

2 תגובות
Why would a cancer cell that has different mechanisms that prevent apoptosis/lysis and promote survival be more affected than a normal cell by stress conditions in the Jolgi system? Why would the cancer cell disintegrate and the normal cell renew the Jolgi system?
Go to hell, the Weizmann Institute, because of you they imposed a blockade on the country and unemployed people