Will stem cells change the definition of "irreversible" injuries and "incurable" diseases? * Update on the US court's decision to cancel Obama's order that allowed public funding for embryonic stem cell research
Update by Avi Blizovsky
A retreat in the US attitude towards stem cells. To the chagrin of President Obama, one of the first things he did when he took office was to cancel the ban imposed by his predecessor George Bush Jr. on public funding for research in the field of stem cells. A federal judge in Washington ruled that federal funding for human stem cell research is illegal.
District Judge Royce Lambert's ruling was made at the request of two Christian doctors who research adult stem cells who asked the court to order the government to outlaw embryonic stem cell research.
According to the judge, in a 15-page decision, the law allowing government funding for stem cell research contravenes the Digley-Vicker Act in that it causes the destruction and destruction of human embryos and thus causes a greater risk of injury or death than those imposed by the law under the rules used in it."
"If one stage of the research results in the destruction of an embryo, the entire project is prevented from receiving federal funding in accordance with the Digley-Vicker Act," writes Judge Lambert.
"Since research on embryonic stem cells requires the separation of these stem cells, the researchers are forced to destroy the embryo," writes the judge. "Therefore, the court concluded that through federal funding for embryonic stem cell research, a violation of the Digley-Vicker Act is caused."
The US Department of Justice announced that it would appeal the offense.
The journal Bio Inform recently conducted a review of the field of stem cells. About two weeks ago we brought the first article in the series "Cells with potential". Here is the second article: "Stem cells in the service of medicine"
By: Judy and Aryeh Melamed-Katz
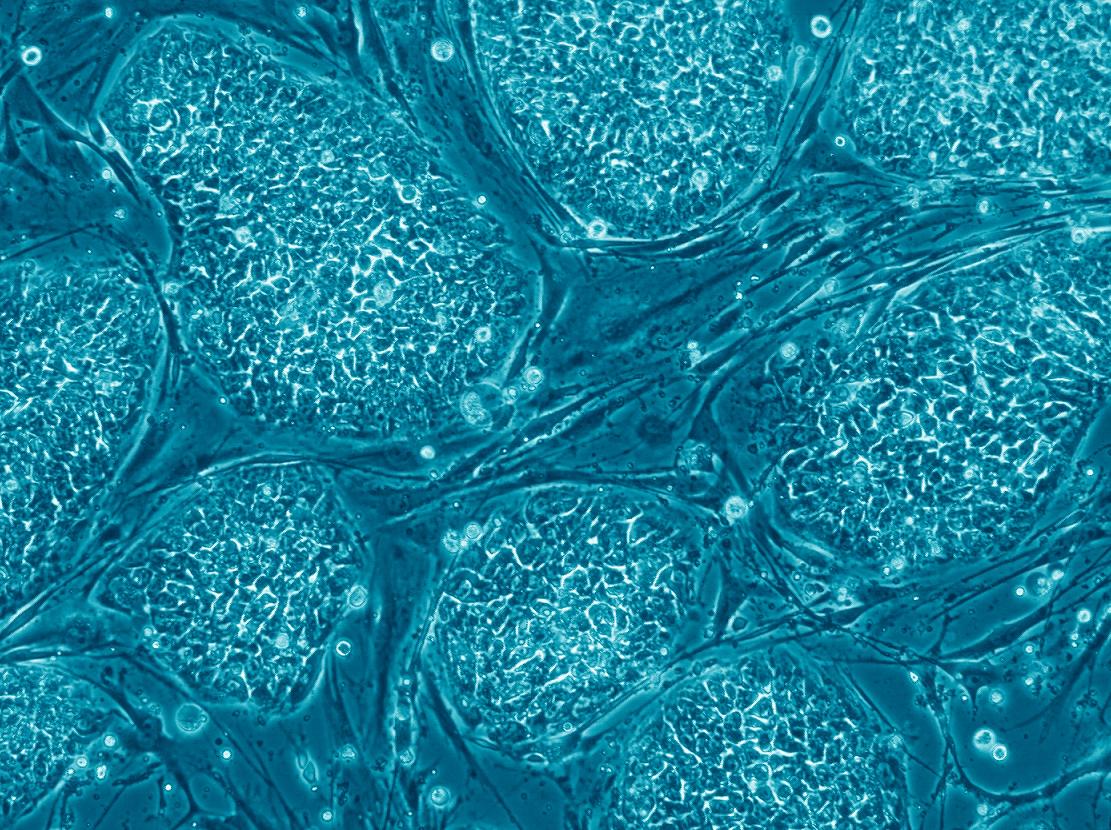
The great promise of the stem cells to the north is their ability to be integrated into a variety of innovative medical treatments, which will allow dealing with diseases for which no cure has been found to date - such as diseases of the nervous system and heart diseases. Lines of human embryonic stem cells and induced pluripotent stem cells (iPS cells) could be used as a source to produce multipotent stem cells, progenitor cells and sorted cells, and these would be transplanted into the patients' bodies. Until now, stem cells of the blood system have been used mainly, derived from bone marrow donation, peripheral blood donation or umbilical cord blood collected at birth, and the extensive experience gained in these transplants could be used in the development of cell transplants derived from pluripotent stem cells. In this article, dedicated to the medical applications of stem cells, we will first review existing treatment methods and later we will deal with some of the future medical applications based on stem cells, which are in various stages of development.
blood system
The origin of the various blood cells in the human body is in the hematopoietic stem cells, found mainly in the bone marrow. Due to the short life span of some blood cells, the blood system needs constant renewal: the body produces on average about one hundred billion new blood cells per day. The hematopoietic stem cells play, therefore, a very important role.
A procedure of transplanting hematopoietic stem cells derived from the bone marrow has been used for 50 years to restore the circulatory system of blood cancer patients and other types of cancer, who were first treated with radiation or chemotherapy in order to destroy the cancer cells. In addition to this, transplantation of hematopoietic stem cells is also used to treat diseases in which the blood system has been damaged, such as thalassemia (a genetic disease characterized by defective hemoglobin), aplastic anemia (characterized by a lack of various blood cells due to a damaged bone marrow) and various autoimmune diseases. Today, tens of thousands of hematopoietic stem cell transplants are performed worldwide every year.
Hematopoietic stem cells are found in large quantities in the bone marrow, and therefore bone marrow transplantation was for years the main means of transferring these stem cells from a healthy person to a sick person. However, bone marrow transplantation is a relatively complex procedure that requires full or local anesthesia of the donor, and therefore there is currently a clear preference for collecting hematopoietic stem cells from the peripheral blood system. In an adult, the stem cells leave the bone marrow in response to a signal that comes through cytokines, small proteins used to communicate between body cells. In order to encourage the transfer of stem cells from the bone marrow to the blood in the donor's body, it is customary to inject him with a substance that damages the parent cells of the blood cells in the circulation and creates a need for the production of new blood cells (for example, cytoxan). In the next step, growth factors, such as G-CSF and KitL, are injected into the donor body, which encourage the release of the stem cells into the blood. Markers such as CD34, which are on the surface of the hematopoietic stem cells and the progenitor cells that have differentiated from them, can be used at this stage to determine the time when the blood will be collected from the donor's body. It is customary to perform the collection when 50-20 cells labeled with CD34 are counted per microliter of blood. The blood collection itself is carried out in a simple procedure similar to a normal blood donation, and this is the great advantage of the method.
Before transferring the blood to the patient's body, the blood can be enriched in hematopoietic stem cells and progenitor cells through cell sorting performed by identifying unique markers such as CD34. This is especially important in autologous (self) blood donation of cancer patients, and may prevent the return of cancer cells to the patient's body. The sorting is done by one of two methods: magnetic sorting or fluorescence sorting (FACS). The magnetic sorting is based on attaching a particle containing iron to an antibody corresponding to a unique molecule on the surface of the cells that you wish to leave in the implant. When the blood donation is flowed near a magnet, these cells are attracted to the magnet and can be collected. This method is suitable for quick sorting of large amounts of blood, but it has a major drawback: it can be used to enrich the blood graft with cells that contain only one marker, so the level of purity is not high, meaning that the resulting blood may contain unwanted cells. The fluorescent sorting is based on attaching fluorescent markers to antibodies, each of which corresponds to one of the unique molecules on the surface of the cells undergoing sorting. The blood cells are passed, one by one, through a collection of laser beams, and the presence of the markers is detected using sensors. With this method it is possible to locate cells with a specific combination of markers, which characterizes the desired stem cells. In this way, a pure cell graft is obtained, but the method is slow and expensive: with the help of FACS, up to 50 cells can be sorted per second, compared to about 10 million cells per second with the magnetic method.
Due to the high cost, currently, hematopoietic stem cell donations are usually not screened at all or screened only for CD34 markers. This limits the effectiveness of the treatment, especially in autologous donation. Even in an allograft there is a significant advantage to sorting with the help of FACS, which filters out the T cells that may cause graft versus host disease, in which the graft attacks the body.
Another source of hematopoietic stem cells is umbilical cord blood, which can be collected at birth (see Keren Sirota's article "Umbilical cord blood: a promise at dawn" in this issue). In the future it will also be possible to use embryonic stem cells or iPS cells, which undergo directed differentiation into hematopoietic stem cells or progenitor cells of the various blood cells. However, it must be assumed that these experiments will not coincide with the first clinical experiments that will be done with cells derived from embryonic stem cells. This is due to the high availability of hematopoietic stem cells from other sources, and due to the difficulty of producing a graft free of pluripotent stem cells, which may cause the appearance of tumors.
On the other hand, it is possible that the hematopoietic stem cells derived from the donation of an adult will be used in the future to produce other body cells, other than blood cells. In recent years, it has become clear that stem cells can go through a process of transdifferentiation in the body, that is, differentiation into cells that are not in their normal chain of differentiation, but the amount of cells that go through this process naturally is low. Today, substances are known that can cause trans-differentiation in stem cell cultures. If it will be possible to use such substances in order to significantly increase the amount of cells undergoing transdifferentiation in the body, it will be possible to use hematopoietic stem cell transplantation for the restoration of various tissues in the body, and not necessarily for the treatment of the blood system. In addition, transdifferentiation of hematopoietic stem cell cultures, which can be obtained through blood donation, relatively easily and without bioethical problems, can be used to produce large amounts of cells that the adult body hardly produces on its own. However, growth of the hematopoietic stem cell culture in the laboratory is more complex than the growth of embryonic stem cells, and at least at this stage the researchers have difficulty simulating the conditions in the bone marrow that allow the regeneration of the hematopoietic stem cells without undergoing differentiation.
The restoration of the blood system through treatment based on stem cells is therefore an old and accepted medical procedure, which is expected to continue to develop thanks to the extensive experience gained to date, thanks to the abundant availability of hematopoietic stem cells and embryonic stem cells, which can also be used as a source of blood system cells, and thanks to a good understanding of the healing process with this method. Increasing the sorting efficiency of the transplant cells and developing methods of personalized medicine are expected to improve the efficiency of the treatment and reduce the risk of rejection.
Nervous System
The lack of a practical possibility to receive a donation of nerve cells or glial cells from a donor's body, and the body's limited ability to regenerate these cells, limit the ability of modern medicine to cure patients with diseases of the nervous system. The treatment currently given to these patients is mostly aimed at minimizing the damage, and is not able to bring about a complete cure. However, treatments based on stem cells, some of which are expected to soon enter the clinical trial phase, may advance the field several steps forward. The hope is that these treatments, based on the transplantation of cells whose function is to replace the damaged cells, will allow partial or complete restoration of the nervous system in diseases that are currently considered incurable.
The first clinical trials in this field will probably include the transplantation of embryonic stem cells that have undergone partial differentiation into progenitor cells of oligodendrocytes in the bodies of patients with severe spinal cord injury. Such a transplant may significantly improve the mobility of these patients, especially if there has been chronic damage to the myelin layer (demyelination) of the axons, and their function is impaired as a result. Due to the danger of developing tumors following the transplantation of cells derived from embryonic stem cells, an effort is currently being made to examine the transplantation of other stem cells, such as bone marrow stromal stem cells (also called mesenchymal stem cells), in the body of The injured spinal cord, in the hope that these stem cells will undergo transdifferentiation in the body. Until now, no satisfactory results have been obtained in experiments conducted on laboratory animals: the amount of nervous system cells obtained following these transplants was relatively low, and their rate of apoptosis (programmed cell death) in the spinal cord was high. Genetic manipulations of genes related to differentiation and apoptosis may improve the efficiency of the procedure and increase the success of the treatment. If the clinical trials on spinal cord victims are successful, this will also be good news for multiple sclerosis patients, an inflammatory disease characterized by demyelination of axons in the nervous system. Transplantation of oligodendrocytes or their progenitor cells may lead to a significant improvement in the condition of patients suffering from this serious disease.
More time will probably pass before medical methods based on stem cells are established as a treatment for diseases characterized by damage to nerve cells (neurons). In this context, a major goal is finding a treatment for Parkinson's disease. Parkinson's disease is caused by the loss of dopamine-producing nerve cells in the brain, which connect the black substance (substantia nigra pars compacta) to the striatum. Attempts to transplant tissue from the ventral midbrain of aborted fetuses into the striatum have had some success, and in some patients an improvement in function has been found. The connection between the improvement in function and the transplant is evidenced by the fact that in the brains of three of the patients who underwent such a transplant, and who died for another reason, dopamine-producing nerve cells were found in the transplant. On the other hand, the condition of some patients worsened, and they suffered from symptoms similar to the side effects of prolonged use of the drug L-DOPA. It goes without saying that there is a practical difficulty in obtaining a large amount of this type of tissue for transplantation, and that the treatment involves serious bioethical problems.
Embryonic stem cells and iPS cells may be used in the future as a source of large amounts of dopamine-producing nerve cells, but the phase of clinical trials in humans is still relatively far away. The difficulty in applying the method stems from the need to minimize the risk of tumor development, and from the need to produce a cell graft that will be successfully integrated into the complex nervous system of the brain. At the same time, efforts are being made to develop treatments that encourage the stem cells in the body to differentiate themselves into dopamine-producing nerve cells, which will take the place of the damaged ones. This is done by injecting growth factors such as TGF, which is known as a factor that affects the early development of the brain and is expressed in a limited amount in the body of an adult. The results of the experiments in laboratory animals were inconclusive, and this form of treatment is also still far from the stage of clinical trials.
Lou Gehrig's disease (ALS) is characterized by the destruction of motor neurons in the spinal cord, and is considered an incurable disease, which usually greatly shortens the lives of patients. Despite the success in producing motoneurons from embryonic stem cells and pluripotent neural stem cells (NSC), there is still a long way to transplant these cells or their progenitor cells so that they will be successfully integrated into the complex nervous system of the human body and replace the dead nerve cells. On the other hand, experiments in laboratory animals have shown that such transplantation is able to prevent or reduce the further destruction of the nerve cells in the spinal cord through the secretion of neurotrophins (neurotrophin) from the transplanted cells. The power of these growth factors to prevent the death of nerve cells, hence their importance.
Stem cells may also play an indirect role in future treatment of Alzheimer's patients. Transplantation of cells that have been genetically modified to express the nerve growth factor (NGF) may improve the condition of Alzheimer's patients, since NGF is known to prevent the death of nerve cells. First clinical trials, conducted with the help of fibroblasts taken from the patients' bodies, were successful. Stem cells may be even more effective in such gene therapy, thanks to two properties that characterize them: they are easy to carry out genetic manipulations, and they have an excellent migration capacity, which will allow them to reach the right place in the brain. Transplanting nerve cells derived from stem cells, with the aim of replacing the damaged cells in the brains of Alzheimer's patients, is considered a more distant goal.
In the next decade, important developments are expected in the introduction of methods based on stem cells to treat diseases and injuries of the nervous system. Besides the future treatments we have described, progress is also possible in the treatment of stroke, brain cancer, Huntington's disease and other medical conditions. However, our limited knowledge of the nervous system and the mechanisms of the diseases affecting it does not allow rapid progress in this important medical field.
Myocardial
Heart diseases are among the main causes of death in the world. Many of them are characterized by ischemia, a lack of blood supply that causes a lack of oxygen in the heart muscle tissue. The lack of oxygen causes the death of heart muscle cells (cardiomyocytes), and may result in life-threatening damage. The body's ability to repair the damage with the help of somatic stem cells is limited, and the drug treatments and most surgical treatments are also unable to bring about the repair of the damaged tissue. Heart transplantation is a good solution for many patients with severe heart failure, but this solution is limited due to the low availability of organs for transplantation and the risk of rejection. The development of a medical procedure of heart muscle cell transplantation, based on stem cells, may save the lives of millions of patients every year. In recent years, the first clinical trials were conducted in the transplantation of various types of somatic stem cells, and the information gathered could be used in the development of methods of transplantation of cells derived from embryonic stem cells or iPS cells.
If there is reasonable blood flow in the patient's body, the cells can be transplanted into the veins, that is, in a relatively simple procedure. However, there is a preference for transplanting into the coronary arteries, which supply blood to the heart itself, due to the proximity of the transplant site to the destination point of the cells. In patients suffering from poor blood flow, the transplant must be performed in the wall of the heart chambers, through cardiac catheterization or open heart surgery. The mode of operation of the transplanted cells has not yet been fully clarified. Today, it is generally accepted that the ability of these cells to differentiate and integrate into the heart muscle is limited, and their main effect comes from their ability to release growth factors that lead to the creation of new blood vessels (angiogenesis) or their ability to encourage the heart's stem cells (cardiac stem cells) to repair the damage.
Over the past 10 years, skeletal myoblasts, which are committed progenitor cells of skeletal muscles, have been transplanted into the bodies of about 100 heart patients. These cells were taken from the patients' bodies, so the transplant did not involve the risk of rejection, but the myoblast cells did not integrate well with the heart muscle cells, and their survival rate after 3 days averaged 7.4%. At the same time, in a way that has not yet been explained, they had a certain positive effect on the condition of the patients. Heart stem cells can also be used in autologous transplantation. These cells, identified through unique markers, have a high healing potential, and experiments in an animal model have indeed shown some success. The main difficulty stems from their small amount in the body and the need to find a way to significantly increase their amount in the laboratory before transplantation.
Clinical trials in which hematopoietic stem cells were transplanted into heart patients showed that in some of them there was a certain improvement in the blood flow capacity of the left ventricle. However, the initial hypothesis regarding a high degree of transdifferentiation in the transplanted stem cells turned out to be wrong. Additional experiments showed that the transplanted hematopoietic stem cells differentiated into blood cells or fused (cell fusion) with heart muscle cells, and did not differentiate into new heart muscle cells. A special interest exists in the transplantation of stromal stem cells. These multipotent stem cells, which differentiate in the body into bone cells, cartilage cells and other cell types, showed in the laboratory the ability to differentiate into cells similar to fetal heart muscle cells when treated with a DNA-demethylating agent called 5-azacytidine. Under other conditions, in the presence of endothelial growth factors, the stromal stem cells differentiated into endothelial cells (cells found in the inner walls of closed spaces in the body, such as the heart and blood vessels). First clinical trials, involving transplantation of stromal stem cells and progenitor cells into endothelial cells, have recently begun.
The field of stem cell transplantation as a treatment for heart diseases is therefore in the clinical trials phase, and the limited success is probably due to the fact that the optimal formula for transplanting cells that will fully integrate into the heart muscle has not yet been found. A different research direction focuses on the development of a bioartificial heart. In 2008, a group led by Doris Taylor (Taylor) from the University of Minnesota succeeded in removing the cells (decellularization) from a rat's heart and inserting cells from other rats into the remaining extracellular matrix. In this way a beating and functioning heart was obtained. It is possible that on the basis of this method of decellularization, or on the basis of a synthetic matrix, it will be possible in the future to create personalized hearts, which will contain stem cells that carry the patient's genetic information.
The medicine of the future
A comprehensive look at the field of medical uses of stem cells reveals that it is divided into "existing" and "future" - when existing is hematopoietic stem cell transplantation, which has been used for years, with great success, to treat blood diseases and various types of cancer. Stem cells from the body of an adult probably have the potential to cure additional diseases, and in the next decade, clinical trials are expected to continue in the transplantation of somatic stem cells, such as hematopoietic stem cells and stromal stem cells, to treat a variety of diseases. When they are finished, we will have a more complete picture of the treatment options with the help of these cells. The picture that is already being painted shows that even when the transplanted cells failed to replace the damaged body cells, they prevented the death of additional cells by excreting growth factors.
At the same time, the first clinical trials are expected to begin in the coming years, based on lines of embryonic stem cells, and later perhaps also iPS cells. A central target for these experiments is diseases of the nervous system, in particular degenerative diseases that medicine still has difficulty dealing with. Tissue engineering is a more distant goal, but the multiple successes in the field of directed differentiation of different stem cells may eventually lead to this dream also becoming a reality.
Boxa: Personalized Medicine and Stem Cells
Personalized medicine is a medical approach according to which the patient's genetic and biochemical information must be taken into account, in order to adjust the necessary treatment for him. Already in the near future we are expected to see more and more drugs, which will be given to groups of patients according to the results of their genetic and biochemical tests. In the field of transplants, there is a special need to adapt the treatment to the patient, due to the risk of rejection due to genetic incompatibility between the transplant cells and the patient's cells. Using cells removed from the patient's body for the purpose of creating a personal line of iPS cells, or a line of embryonic stem cells derived from medical cloning (see our article "Cells with potential" in this issue), will provide a complete solution to the rejection problem, because the genetic information in the transplanted cells will be identical to the genetic information in the cells the patient's body. These methods may eliminate the need for stem cell banks, because the procedure for creating a personal cell line in case of need is expected to be simple, fast and probably also cheap.
Credit
Judy Melamed-Katz is a chemist completing doctoral studies in microbiology at the Hebrew University; Worked at Teva and MZP, and currently engages in writing and scientific education.
Aryeh Melamed-Katz is an electronics engineer and doctor of physics, a graduate of the Weizmann Institute of Science; Currently engaged in writing, developing science education programs, lecturing on science topics and providing scientific consulting services.
www.arie-science.blogspot.com
ariejudy@gmail.com
for further reading
Department of Health and Human Services, "Regenerative Medicine", August 2006.
http://stemcells.nih.gov/info/2006report/
Powell, JL, Hingorani, P., Grupp, SA and Kolb, EA, “Hematopoietic Stem Cell Transplantation”, eMedicine.
http://emedicine.medscape.com/article/991032-overview
Kim, SU and de Vellis, J., “Stem Cell-Based Cell Therapy in Neurological Diseases: A Review”, Journal of Neuroscience Research 87(10):2183-2200 (2009).
http://www.ncbi.nlm.nih.gov/pubmed/19301431
hESC-derived Oligodendrocytes by Geron:
http://www.geron.com/products/productinformation/spinalcordinjury.aspx
Lindvall, O. and Kokaia, Z., "Prospects of Stem Cell Therapy for Replacing Dopamine Neurons in Parkinson's Disease", Trends in Pharmacological Sciences 30(5):260-267 (2009).
http://www.ncbi.nlm.nih.gov/pubmed/19362379
Lee, J. and Terracciano, CM, “Cell Therapy for Cardiac Repair”, British Medical Bulletin 94(1):65-80 (2010).
http://www.ncbi.nlm.nih.gov/pubmed/20200014
Taylor, DA, "From Stem Cells and Cadaveric Matrix to Engineered Organs", Current Opinion in Biotechnology 20(5):598-605 (2009).
http://www.ncbi.nlm.nih.gov/pubmed/19914057

3 תגובות
To 2:
There is also a much simpler option to grow stem cells, and it actually involves the destruction of only one embryo:
The stem cells in the first days of the embryo (harvesting at the ideal time occurs approximately 5~7 days after fertilization, which is done in vitro, or in vitro fertilization), multiply and shrink all the time, because there is no room inside the egg, which is the shell for reproduction.
Take the stem cells out of the egg's protective shell (for the future placenta), and they will continue to reproduce endlessly, trying to fill the space.
I don't know, maybe it's a tumor in a liquid medium like you said. I saw it in a program a few years ago that dealt with the usefulness of the thing.
In addition, the USA is one of the dumbest countries in terms of legislation.
Notice what the "embryo" lacks in the first week of its "life" (the ideal period of harvesting the cells)
heart
brain
respiratory system
digestive system
reproductive system
In fact, I believe that these five characteristics represent all the criteria that biologists have defined for living production.
Meaning, the "embryo" in the first week, despite its potential to become a human, is less than a bacterium in the sense of:
He is an inanimate object.
And although I hate to involve religion in any issue related to medical or technological development (I'm an atheist myself), but at least the Jews have some sense (it's not just that they are among the most successful nations. It's a shame that this is only expressed in the diaspora =/), and they set their standard for life only when the fetus A brain begins to grow.
I would like to make two comments:
beginning:
The law in question (Dickie Wicker) is an example of ill-considered, harmful and ultimately immoral legislation - based on irrational 'moral' dogmas.
The basic problem in the issue of embryos is the question of what is that creature that is a being that carries the right to life.
The Christian view, very simplistic, holds that every fetus is like that - without paying attention to the degree of its development.
The liberal approach, which is also simplistic, holds that a fetus does not bear rights, including the right to life, as long as it is not actually born.
Both approaches are based on purely technical distinctions: the Christian one - on the very existence of a collection of cells with development potential, called an embryo, even if it is devoid of any consciousness and even if it is devoid of any functional qualities; And the liberal one - on the fact that the fetus at every stage is physically 'connected' to its mother, even if it has consciousness and if it has actual functional capacity outside its mother's womb.
Both approaches lead to harmful and immoral legislation: the Christian one - in the form of legislation of the type of the Dickie and Wicker Law - in that it prevents vital scientific development and harms humanity's chances of healing from diseases and accident damages that cannot be dealt with except in ways based on stem cell methods and technologies. The liberal - in that it leads to the massive killing of fetuses that have consciousness and even actual functional skills.
In my opinion, a fundamental view is needed on this issue. For thousands of years, Jewish law has offered a meaningful distinction, which relates to the maturity of the fetus, in that it denies the existence of a fetus as a bearer of life before it is 41 days old, but takes a principled position (which has certain qualifications) according to which the fetus bears the right to life from this age onwards. Today we clearly know that the brain of a fetus works for the first time electrically starting from the 41st day, so this stage of development and at this time can be seen as an essential marker regarding the existence of a fetus with primary consciousness.
It seems to me that if the development of an embryo is frozen in such a way that it does not reach this stage of brain activity, there is no reason to fear any moral flaw in the use of its stem cells or the destruction of the embryo, at any time and at any age. In any case, even without freezing the development of the fetus as above, it will be possible to use it without any hindrance or moral entanglement, until the 41st day, since at this stage of its 'life' the fetus cannot have any legal personality, since it lacks minimal cognitive capacity .
Second:
It is not at all clear to me why, with regard to the rejection of the order by the High Court, no distinctions were made between research involving the destruction of embryos, and research methods dealing with the 'industrial' growth of stem cells (for example - in a liquid medium, as recently demonstrated in Israel). The short-sightedness of the court seems puzzling and worrying, against the background of the rationale of the law, and there is here the addition of the sin of stupidity to the crime of immorality.
The law in question is called the Dickey-Wicker Amendment.
See here:
http://en.wikipedia.org/wiki/Dickey_Amendment