Nature assigned to the embryonic stem cells a huge, almost unimaginable potential - the creation of a complete living being, with all its organs and functions. Today, scientists assign another important role to these cells: to be a medical tool that will restore degenerated cells and damaged tissues

Nature assigned to the embryonic stem cells a huge, almost unimaginable potential - the creation of a complete living being, with all its organs and functions. Today, scientists assign another important role to these cells: to be a medical tool that will restore degenerated cells and damaged tissues, and will be used to cure severe genetic diseases - such as autoimmune diseases and degenerative diseases of the nervous system. "The main obstacle currently preventing the use of stem cells in medicine is the difficulty of getting the stem cell to differentiate into the desired cell in an efficient and satisfactory manner; We still don't know how to reproduce this process accurately," says Dr. Yaacov Hana, who recently joined the department of molecular genetics at the institute. In his new laboratory, he and the members of his group are trying to understand the biology of these special cells, with the aim of answering basic questions concerning the mechanisms of development and differentiation, and promoting their use as a powerful medical tool.
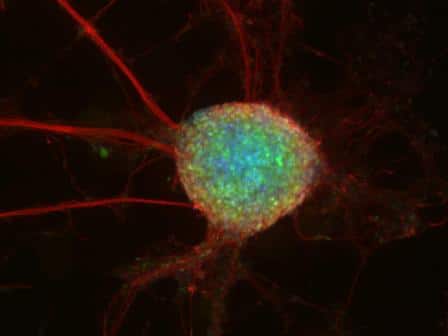
Embryonic stem cells are unsorted cells that carry the potential to become any cell in the living body. During embryonic development, they go through a sequence of developmental stages, which are under close and gentle control, when decisions regarding their future have to be made quickly and with exemplary precision - any mistake leads to disaster. For the scientists, this is a treasure, since these cells allow them to reproduce complete developmental processes - in a laboratory dish. The cells are actually "frozen" in time, in an unsorted state. Dr. Hana grows them under different conditions, and performs genetic and other manipulations on them, in order to try to answer various questions: How do the embryonic stem cells maintain their developmental potential? How is the subtle and complex control of their differentiation done? How is the fateful decision made quickly whether to differentiate or not to differentiate? And also: what can be learned from these experiments - carried out in a dish - about what happens in the embryo, and in particular in the human embryo?
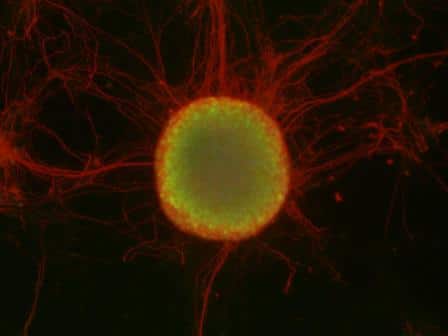
A colony of stem cells (in the center, in green), surrounded by nerve cells (in red)
journey to the past
Another aspect of stem cell research that interests Dr. Hana is related to a scientific breakthrough of a few years, which occurred when scientists were able to take a mature and sorted skin cell, and reprogram it so that it becomes a perfect embryonic cell again; A kind of "journey to the past" in developmental terms. Surprisingly, the return of the cells to an initial developmental stage is possible as a result of the introduction of only four genes into the genome of the adult cell. The possibility of producing stem cells in the laboratory solves the ethical problems associated with the use of embryonic stem cells - which originate from eggs or fertilized eggs. Another promise inherent in the method is that in this way it will be possible to treat patients with genetic diseases without the need to find a suitable donor: the cells will be taken from the patient himself, and reprogrammed into stem cells. After that, it will be possible, through genetic engineering, to repair the damaged genes. The repaired stem cells will undergo differentiation into the desired cell type, and will be transplanted back into the patient.
In his post-doctoral research, Dr. Hana and his research partners were the first to demonstrate the feasibility of the method, when they were able to cure a mouse with sickle cell anemia. In another study - the purpose of which was to check why only a small percentage of mature cells successfully go through the reprogramming - he discovered that there are several main "switches", and that the process is only complete if they are all turned on. Following these discoveries, he is now interested in better understanding how one cell turns into another cell, what are the genes and mechanisms involved in the reprogramming process, and what is the exact role of the switches - and why they are so essential. Another interesting point is that, while the scientists struggle to reprogram mature cells, with relatively small success rates, the egg actually knows how to carry out the process with perfect success. In his research, Dr. Hana plans to try to understand how she does it.
Besides the aspiration to understand basic mechanisms of differentiation and development processes, and the promise that they bear in the field of rehabilitation medicine, stem cell research could provide a modern and effective tool for the study of genetic diseases in humans. These diseases - such as type 1 diabetes, Parkinson's, Alzheimer's and more - usually cause the destruction and degeneration of the damaged cells, which makes it difficult to study the disease. Dr. Hanna plans to use the reprogramming process to produce stem cells derived from mature cells of patients with genetic diseases. On these cells it will be possible to use the various research methods of genetic engineering: insertion or deletion of genes, insertion of markers, and more. In this way, an effective model for the study of genetic diseases will be obtained - which will also make it possible to search for cures for these serious diseases.
personal
Yaakov Hana was born and raised in the village of Rama in the Galilee to a family of doctors - his grandfather, father and three sisters are doctors. Loyal to the family tradition, he studied medicine at the Hebrew University in Jerusalem, but found himself drawn to research, and preferred spending time in the laboratory to treating patients. "My uncle, who is a scientist and director of a pharmaceutical company, and was behind the development of the first antibody approved for the treatment of lymphoma in humans, was the one who influenced and gave the inspiration." In 2007 he completed his studies with honors in a combined path of doctors-researchers (PhD/MD). He then embarked on post-doctoral research in the group of Prof. Rudolf Janisch, at the Whitehead Institute for Biomedical Research at the Massachusetts Institute of Technology (MIT). His research won him many awards, including, in 2010, an award for young inventors (under the age of 35), awarded on behalf of MIT's Technology Review magazine. In 2011 he joined the faculty of the Department of Molecular Genetics at the Weizmann Institute of Science.
Yaakov Hana lives in Tel Aviv. He devotes his free time to his favorite hobby - scientific research. But in addition to that he manages a Tel Aviv bar, together with three good friends.

4 תגובות
I'm actually with Michael.
I read, thanks.
By the way, I still maintain that the presumption is wrong.
The people:
I suggest you read my response:
https://www.hayadan.org.il/sex-as-we-know-it-works-thanks-to-ever-evolving-host-parasite-relationships-0711/#comment-301341
Nature intended??? What a religious title