Scientists have succeeded in developing an innovative method with which it is possible to "look" at proteins while they fold - in less than a millisecond - into the elaborate and convoluted shapes that determine their activity.
Humans have about thirty thousand genes, responsible for the creation of more than two million proteins. The protein molecules are the ones that do most of the work in the cells.
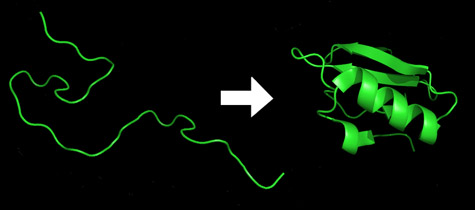
Proteins are obtained as straight chains of amino acids that fold independently to their so-called "natural form" within a few milliseconds/seconds. The activity of the protein strongly depends on its final form. For example, enzymes and the molecules they change are often described as fitting together like a lock and its key. By the same logic, misfolded proteins are responsible for a number of neurodegenerative diseases of the worst kind, such as Alzheimer's disease, Parkinson's disease and mad cow disease.
Existing test methods are unable to match the speed at which the proteins fold. Predicting the folding form of the proteins without prior knowledge requires the use of powerful supercomputers or the method of "cloud computing" (using the shared computing power of a large number of home computers). Either way, prediction is a lengthy and sometimes imprecise method, and as a result, the delay in understanding the relationship between a protein and its structure slows down the utilization of DNA sequence information in the fields of medicine and biotechnology. A sophisticated method for observing the processes in which proteins fold and unfold may, in the end, provide the kind of details needed to improve the quality of protein structure prediction.
In the latest issue of the online version of the Journal of the American Chemical Society, three scientists, led by Dr. Michael L. Gross, professor of chemistry at Washington University in St. Louis, describe preliminary research in which they used an innovative approach to observe the folding of Small proteins called barstar.
Their method is similar, in a general way, to the method by which flying projectiles or exploding balloons are photographed today using a stroboscope (a device for tracking objects in cyclic motion) and a high-speed camera. The scientists use a parallel version of this well-known method to "watch" folding proteins. The "strobe light" in this case is a jump in temperature and the "camera" is a chemical reaction whose result is measured using a sensitive mass spectrometer.
"Imagine the protein as a sequence of thousands of atoms connected together by springs," explains the lead researcher. "If we hang this chain from the ceiling with a string and let it swing, imagine the sheer number of shapes that will result."
The natural form of barstar proteins and their amino acid sequence are known and recognized. What is not known is how the sequence twists and folds into its final structural form. Fortunately for the scientists, barstar proteins, unlike most proteins, do not fold at a temperature of zero degrees Celsius, and they begin to fold only after they are heated. The folding itself takes only microseconds.
The scientists began the test by injecting a cold solution of the proteins and a small amount of hydrogen peroxide into a tiny, hollow optical fiber. In the next step, tiny lumps of the sample in the fiber are heated using fast laser pulses. The first pulse heats the solution to the point where a particular protein conformation is energetically favored. The second pulse breaks down some of the hydrogen peroxide molecules into two halves, each of which constitutes a highly active radical (hydroxyl). These radicals react quickly with the parts of the protein exposed to the solution, and "mark" them with oxygen atoms. Radical reactions must be completed very quickly; Otherwise, some of the marking will occur in the internal parts of the structure. Within microseconds, amino acids that act as radical scavengers neutralize any excess radicals that remain, to prevent them from breaking bonds or changing the shape of the protein. This process is repeated over and over about five hundred times, while obtaining "pictures" of the track where the protein rapidly changes its configuration. "The radicals do not mark all the atoms," notes the lead researcher. "However, they mark about half of the amino acids, a pretty good result. Most other chemicals are too picky or too slow for this type of experiment." "We collect every drop of labeled protein as it leaves the fiber," explains the researcher.
"In the next step, we cut the protein slowly and carefully using an enzyme that breaks down the amino acid chains at defined and predetermined points, to obtain known protein segments, known as peptides. These protein segments are next separated according to their nature using liquid chromatography, and a mass spectrometer then "weighs" the amount of each of these types to see if they are labeled with oxygen or not. "Using the same device, we continue and break down the protein segments again and again and check if they contain the labeled oxygen."
"Since we use a chemical and not a physical test gauge, we are able to examine the process in more detail," explains the researcher. "We are able to determine which part of the final structure folds first, which second and so on."

2 תגובות
I just read a long article by Prof. Doron Orbach on the website "Hados" and it made me sad.
The battle for humanity's sanity is long and hard and we seem to be losing it.
We need to establish an atheist state on some island in the Pacific Ocean and leave humanity to sort itself out.
I recommend 2 excellent courses on biology and brain research, in the first one the topic of protein folding is also nicely explained:
http://www.youtube.com/view_play_list?p=ACC83028E0CBD671
http://www.youtube.com/view_play_list?p=D8C99F67C81778E8