The innovative anode could assist in the development of a safe lithium battery with a higher energy capacity
[Translation by Dr. Nachmani Moshe]
Researchers have succeeded in developing an anode based on lithium metal in its semi-liquid form in what represents a new paradigm in the field of battery development. Lithium batteries made of this new type of electrode could have a higher capacity and be much safer than ordinary lithium batteries. The researchers published their research findings in the scientific journal Joule.
Lithium-based batteries are one of the most common types of rechargeable batteries used in modern electronic components, thanks to their ability to store large amounts of energy. Normally, these batteries contain flammable liquid electrolytes and two electrodes - anode and cathode - separated by a membrane. After the battery goes through repeated charges and discharges, deposits of lithium called dendrites may develop on the surface of the electrode. These deposits may puncture the membrane that separates the electrodes and allow them to react with each other to the point of shorting the electrical circuit, and in worse cases even lead to causing a fire. "Incorporating a metallic lithium anode into batteries based on lithium ions has the theoretical potential to create a battery with a greater capacity than a battery with a graphite anode," explains the lead researcher. "However, the most important factor we need to take care of is that the battery we created will be safe."
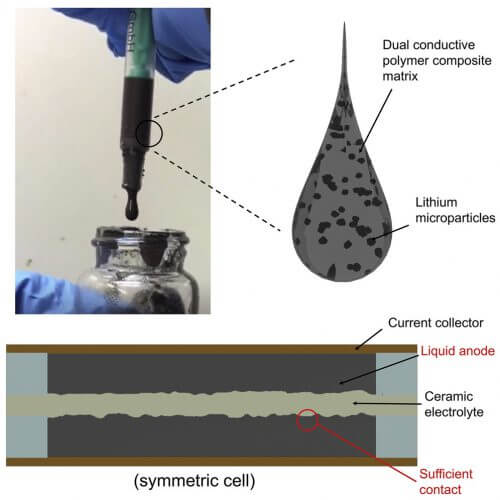
One of the proposed solutions to the problem of flammable liquid electrolytes used in batteries today is to convert them into solid ceramic electrolytes. These electrolytes are highly conductive, non-flammable and stable enough so that dendrites will not form. However, researchers found that the point of contact between the ceramic electrolyte and a solid lithium anode is not sufficient to store and supply the amount of energy required for most electronic components. The researchers were able to overcome this setback by creating a new family of materials that can be used as a semi-liquid metallic anode - they were able to develop a double-layer conductive polymer/carbon composite substrate containing lithium microparticles uniformly distributed along the length and width of the substrate. At room temperature, the substrate can flow like a liquid and thus it allows sufficient contact with the solid electrolyte.
By combining the semi-liquid metal anode with a solid ceramic electrolyte based on the mineral garnet, the researchers were able to achieve a current density ten times higher than cells with a solid electrolyte together with a normal lithium anode. This cell had a much longer life cycle than normal cells. "This innovative process leads to obtaining a battery anode based on lithium metal that can flow and has extremely good levels of safety and performance compared to normal lithium metal. The application of such a new material could lead to a conceptual change in lithium-based rechargeable batteries, and we are working hard to see how this system will work in a variety of battery designs," explains the lead researcher.
The researchers believe that their innovative method could have a significant impact. For example, the system could be used to create high-capacity batteries for electric vehicles and special batteries for use in wearable devices that require flexible batteries. The researchers also believe that their methods can be used beyond lithium metal in other systems of rechargeable batteries, including batteries based on the metals sodium and potassium, which can be used to store electricity in the systems of the national electricity grid.
More of the topic in Hayadan:

2 תגובות
Why did the researchers spend tens of millions of dollars developing a "double-layer polymer/carbon conductive composite substrate containing lithium microparticles uniformly distributed along the length and width of the substrate"? After all, it is much simpler to use amalgams of lithium/mercury or lithium/gallium - both of which are very cheap, liquid (gallium melts at around 35 degrees Celsius), have slight and slow temperature changes during use (because gallium and mercury have a high specific heat capacity), conduct Excellent electricity, allowing a homogeneous dispersion of lithium (both atomic and ionic) in a variety of extremely high concentrations - and finally: the risks of using these amalgams, as well as the defense techniques in the event of their release from their cases are well known and varied.
And what about an explanation of what semi-liquid is?