Researchers from the Carnegie Institute's Geophysical Laboratory report changes in the melting temperature of solid nitrogen at a pressure of 120 gigapascals (over a million atmospheres) and at a temperature of 2200 degrees Celsius
Nitrogen atoms prefer to exist as pairs, joined together by one of the strongest chemical bonds in nature. By exposing nitrogen compounds to extreme temperatures and pressures, scientists were able to gain new insights not only about this compound but also about similar compounds, such as hydrogen.
In the current electronic issue of the journal Physical Review Letters, researchers from the Carnegie Institute's Geophysical Laboratory report on changes in the melting temperature of solid nitrogen at a pressure of 120 gigapascals (over one million atmospheres) and at a temperature of 2200 degrees Celsius.
These results, in addition to the visible changes in the structure of solid nitrogen at high pressure, could lead to future innovative types of nitrogen- or hydrogen-based fuels with high energy content. Theoretical polymers of nitrogen could lead to materials with a higher energy content than any other known material.
The researchers from the Carnegie Institution and their colleagues from the Lawrence Livermore National Laboratory compressed liquid nitrogen in a device known as a "diamond anvil cell" that produces enormous pressures by compressing the sample between two high-quality diamond stones. Since diamonds are transparent to most wavelengths of visible light, the sample can be heated during the experiment with a laser. A method known as Raman spectroscopy uses the radiation emitted from the heated sample to diagnose and identify the changes in its molecular structure as they occur.
"To date, no researcher has made observations at the site of the occurrence itself regarding the behavior of nitrogen under such extreme conditions of temperature and pressure," says the lead researcher. "Our measurements regarding the melting values and vibration characteristics of the liquid, as obtained by Raman spectroscopy, give us a very clear picture of the behavior of the nitrogen atoms and the molecular bonds between them under these conditions."
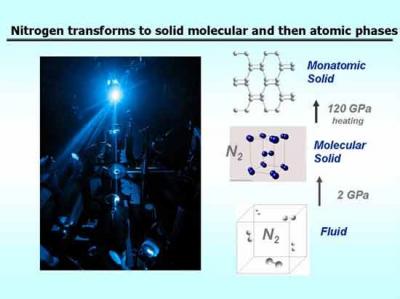
A diagram of the temperatures and pressures at which a substance changes its state of aggregation (from liquid to gas or from one crystalline structure to another, etc.) is called a phase diagram. For nitrogen, similar to most other substances, the high temperature and high pressure regions of the phase diagram are areas that have hardly been explored. Researchers hope that these unknown complexes will be important starting points for new materials with useful properties.
At room temperature and atmospheric pressure, nitrogen is a gas, but it can be cooled and compressed into a liquid or solid form depending on the temperature and pressure applied to it. Even as it passes between the different phases, nitrogen remains a diatomic compound consisting of two atoms connected by a strong and high-energy chemical bond - a triple bond.
"Nitrogen compounds tend to be energy-dense substances," says the lead researcher. "Pure nitrogen could be a fuel or a powerful explosive if we could figure out how to bond nitrogen atoms to the material in a way other than diatomic splitting in a triple bond. Recent studies have been able to show that nitrogen becomes a non-dissociated state with a single bond rather than a triple bond at very high pressures. These could be used as high-energy materials if they could be held at normal pressures. The findings of our research will be able to help demonstrate the way to prepare these materials under much less extreme conditions."
Filling the gaps in the nitrogen phase diagram has important implications for the study of other essential substances, the researcher explains. "Nitrogen is a prototype for a diatomic atom. Knowing its phase diagram and other properties can indicate the behavior of other diatomic substances, mainly hydrogen. Many key transformations as well as other phenomena occur in nitrogen at much lower pressures than those of hydrogen," he explains. "Hydrogen is the fuel of the future. In theory, hydrogen could have very interesting properties at high pressures, among them metallic behavior, and the existence of superconducting and liquid states (superfluid). The question of whether it is possible to obtain materials with such properties and preserve them under normal pressures is still open. However, thanks to the research on nitrogen, we are moving forward quickly towards solving this question."

3 תגובות
I need a graph of nitrogen behavior in changing temp.
Greetings,
Did you ask me?
Is it possible to put the nitrogen into a small container similar to a domestic air outline?
Can it be used by "spraying" action?
How dangerous is this substance to human health? the animal? And does it harm the quality of the environment or is it friendly?
I would love to receive your answer,
Thanks and happy holiday
Peace,
The message is for Dr. Moshe Nachmani.
I would appreciate it if you could direct me how to get to the original article or at least the name of the researchers.
with gratitude
Inhorn Yossi