The ubiquitin system, for which Prof. Chachanover and Prof. Avraham Hershko won the Nobel Prize, creates a protein that dramatically inhibits the development of cancerous tumors

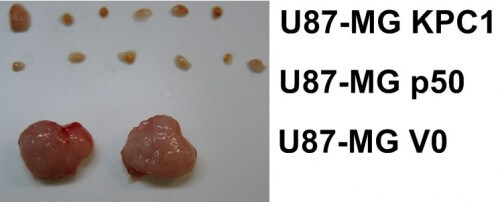
A new article by 2004 Nobel Laureate in Chemistry Research Professor Aharon Chachanover, a faculty member at the Rappaport Faculty of Medicine at the Technion, was published on April 9 in CELL - the most important journal in the life sciences - and reports on the discovery of two suppressive proteins -cancer. In the picture below you can see the dramatic effect of these proteins on the cancer tumor: above the two lower tumors (the control group) are visible cancerous tissues in which a high level of the same proteins was administered.
The research was done in the laboratory of research professor Aharon Chachanover, a faculty member at the Rappaport Faculty of Medicine at the Technion. Professor Chechenover won the 2004 Nobel Prize in Chemistry (together with Professors Hershko - also from the Technion - and Rose) for the discovery of the ubiquitin system, and the current study is a continuation of that discovery. The research was led by research associate Dr. Yelena Kravtsova Ivantsev, and was partnered by students and other research associates and doctors from the Rambam, Carmel, and Hadassah medical centers who are involved in tumor research and treatment.
The ubiquitin system is an important and essential component of cell life, as it is responsible for the breakdown of disordered proteins that may harm the cell and tissue. This system tags these proteins and sends them to be broken down in a protein complex called the proteasome.
In general, the proteins that reach the proteasome are completely degraded, but there are exceptions, and in the current study the precursor p105 was examined. It turns out that p105 is indeed broken down in some cases following its tagging by the ubiquitin system, but in other cases it is only cut and shortened and becomes a protein called p50.
p50 is one of the components of the NF-κB protein - found to be the factor linking inflammation to cancer. The hypothesis of the connection between inflammatory processes and cancer was first put forward in 1863 by the German pathologist Rudolf Wircho, and has been proven over the years in a series of studies. Since the discovery of NF-κB nearly three decades ago, thousands of articles have been published linking it to the development of cancerous tumors. This protein is involved in malignant tumors of various types (prostate, breast, lung, head and neck, colon, brain, etc.) in several parallel ways: NF-κB [a] inhibits apoptosis (programmed cell death) which is essential for normal body activity; [b] accelerates the uncontrolled division of cancer cells; [c] helps in the creation of new blood vessels (angiogenesis) essential for the tumor, and [d] enhances the resistance of the cancer cells to radiation treatments and chemotherapy.
As mentioned, the precursor p105 (related to NF-kB) is 'handled' by the ubiquitin system in one of two ways that work simultaneously and are in equilibrium: [1] degradation or [2] shortening and conversion to p50. The current study deciphers the decisive 'decision-making mechanism' between the two possibilities: when a component of the ubiquitin system called KPC1 is involved in the process and attaches ubiquitin to p105, the protein will be saved from degradation and become p50. When ubiquitination occurs without KPC1, p105 will be degraded.
Deciding between these two options has dramatic consequences for the cell, since a high presence of KPC1 (which leads to the said process) and p50 (the product of the process), while violating the normal balance between the processes, suppresses the cancer tumor and protects the healthy tissue. In the current study, which was done in human tumor models grown in mice, but also in human tumor samples, a strong connection between suppression of malignancy and these two proteins was discovered, and therefore it is clear that increasing the presence of p50 in the tissue may protect it from cancerous tumors.
Professor Chechenover, who also serves as president of the Cancer Society, points out that "many more years are needed to establish the research and to fully understand the mechanisms behind the suppression of tumors. Developing a drug based on the discovery is a possibility, although not certain, and in any case the road to get there is long and not easy."

3 תגובות
Go forth and succeed
Urgently - another Nobel Prize!! Well done - the savior of humanity!