Israeli scientists and doctors have developed a new method for the early detection of blood cancer of the myeloma type, which will allow targeted treatment according to new and unique genetic characteristics of the tumor cells
Malignant diseases develop when cells lose control and multiply uncontrollably. Research efforts worldwide are aimed at deciphering the "master plan" of cancer cells - and understanding how they take over growth pathways that help them reproduce and spread in an uncontrolled manner. Understanding these mechanisms may help develop effective ways to fight the disease.
Collaboration between scientists from the Weizmann Institute of Science and doctors from the hemato-oncology departments in Israel, led to the precise identification of the "personal" genetic profiles of multiple myeloma blood cancer cells. As part of the joint research, genetic profiles of the cancer cells were identified in four groups of patients: patients who were in a pre-cancerous stage, myeloma patients, patients after treatment - and patients who were treated, responded to treatment, but their disease returned and became active (relapse). The scientists and doctors estimate that the personal genetic mapping will help in the future in the development of new methods for the diagnosis and targeted treatment of this serious disease within the framework of "personalized medicine".
Multiple myeloma is the second most common blood cancer. It develops when plasma cells - the cells that produce antibodies in the bone marrow - multiply uncontrollably and lead to system failure and death. The disease has been studied for many years, and in recent years a significant improvement has been achieved in the survival of patients with it - through immunotherapy treatments; But in most patients the disease relapses. One of the main obstacles in the treatment of myeloma is the great variation between the patients with the disease and the difficulty of diagnosing the patients in its early stages and adapting the optimal treatment to them.
The plasma cells of the control group were very similar to each other, and presented a uniform and normal genetic profile of normal plasma cells. Comparing the normal pattern to the patterns identified in the patients, showed that each patient has a unique genetic pattern"
For example, patients who are randomly identified in a routine blood test as blue in the pre-cancerous stage of the disease, remain under medical surveillance without treatment (watch and wait). Every year about 1% of them develop the disease, but until the current study, there was no way to differentiate between those who will develop myeloma, and those who will not develop the disease.
Dr. Guy Ledergor, a physician and researcher, and Dr. Assaf Weiner from Prof. Ido Amit's research group in the Department of Immunology at the Weizmann Institute of Science, together with the research group of Prof. Amos Tani from the Department of Computer Science and Applied Mathematics at the Weizmann Institute of Science, believed that Ritzoff Single-cell RNA sequencing - an extremely sensitive method developed by the members of the group - may allow a new approach to understanding the causes of multiple myeloma outbreaks as well as to the diagnosis and treatment of the disease.
The set up for scientific-clinical collaboration at the Weizmann Institute of Science, headed by Prof. Gabi Barbash, helped recruit all hemato-oncology departments in Israel to collaborate with the institute's scientists in this unique research.
The new research method allows researchers to sequence RNA in thousands of individual cells from the patient's blood, or from his bone marrow, and thus obtain the genetic profile of RNA in each individual cell. By sequencing tens of thousands of cells from healthy people who had undergone hip replacement surgeries, the scientists initially documented the "normal profile" of normal plasma cells; The healthy group was the control group. The plasma cells of the control group were very similar to each other, and presented a uniform and normal genetic profile of normal plasma cells. Comparing the normal pattern to the patterns identified in the patients, showed that each patient has a unique genetic pattern - in some patients several "clones" of cells with different genetic profiles were observed in the same patient.
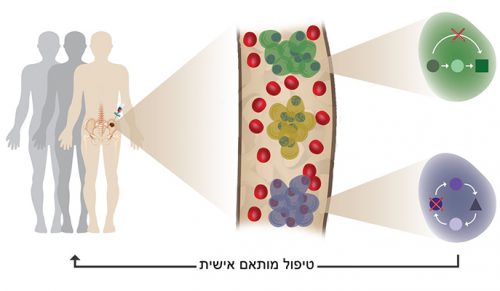
With the unique method developed in the research, even a very small number of malignant cells can be detected in the blood, and this, even at a very early, pre-cancerous disease stage. Detecting cells in a blood test could replace the invasive (and painful) bone marrow biopsy for monitoring these patients. A simple blood test will allow early detection, accurate diagnosis and more informed decisions regarding treatment, based on the "personal" disease profile of each patient.
The research and the unique and sensitive genetic analysis, which was applied in this research for the first time in the world, are published today in the scientific journal Nature Medicine, and will help advance Israeli medicine at the forefront of treating myeloma patients.
The identification of the rare cancer cells and their characterization was made possible through the methods of artificial intelligence and big data, and this is the direction that future medicine will take more and more.
Prof. Barbash points out that the current achievement is only the beginning in the application of RNA sequencing technologies in single cells in clinical-genomic studies. "We are now involved in the development of similar collaborations between researchers in hospitals and Weizmann Institute of Science scientists, with the aim of promoting understanding of mechanisms, diagnosis and treatment of other diseases: from cancer and autoimmune diseases to Alzheimer's."
"Genome analysis in a single cell has so far been limited to a limited number of research laboratories," says Prof. Amit. "We are constantly expanding the limits of technology, so that in the future it can become an important clinical tool for diagnosing and monitoring many malignant diseases in the pre-cancerous stage, or for monitoring their re-emergence after oncology treatment. This technology will also contribute to the development of new and more effective immunotherapy treatments, based on the huge database built on the disease."
"The identification of the rare cancer cells and their characterization was made possible through the methods of artificial intelligence and big data (huge data), and this is the direction in which future medicine will move more and more," says Dr. Assaf Viner.
"In the future," says Dr. Ledergor, "doctors will be able to monitor the disease in real time and treat each patient according to their individual disease profile, perhaps even before the first signs appear."
More of the topic in Hayadan:
- Cancer treatment is a double-edged sword
- Researchers succeeded in producing particles to transport new drugs and doubled the lifespan of mice with human lymphoma
- A surprise at the ambassador's house: a bone marrow cancer patient being treated with a drug derived from the discovery of the Nobel laureates
