Weizmann Institute of Science scientists have shown that disordered proteins adapt well to environmental changes in the cell and form resistant networks of proteins that work together and thereby cover up the lack of organization
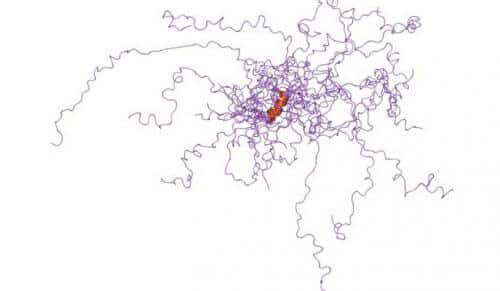
The very name "disordered proteins" implies that there is something wrong with proteins that are not neatly folded into three-dimensional structures, but instead sag freely like noodles. A new study by Weizmann Institute of Science scientists, recently published in the "Records of the American Academy of Sciences" (PNAS), points to the advantages of these proteins: they adapt well to environmental changes inside the cell, and the lack of organization does not prevent them from functioning well.
"Evolutionarily more developed organisms have more disordered proteins compared to the amount of these proteins in bacteria," says Dr. Hagen Hoffman from the Department of Structural Biology. "The fact that these proteins gained a foothold at a relatively late stage of evolution may suggest complexity and sophistication, but what exactly does nature need them for?"

When disordered segments of protein molecules were first discovered in the late 80s, scientists used to cut them out and throw them away, because it was impossible to make them into crystals and study their structure using crystallography. However, over time, it became clear that the disordered state is much more common than first thought: about a third of the proteins in the organism are disordered to one degree or another; In many of them only a certain part of the molecule is disordered and in others - in the entire molecule. It was also discovered that disordered proteins are apparently an important type of biological molecules that participate in the regulation of gene activity and other essential functions. Moreover, it is possible that under certain conditions they have an advantage over ordered proteins: they are easier to change or disassemble when the environment changes, and they may be more suitable for carrying out different tasks, since their structure is more open, literally, to connect with other molecules compared to the folded structure that requires precise structural adjustment .
gene expression
But if disordered proteins are so flexible in structure and function, are they even durable enough to perform important tasks? Dr. Rene Vankranenbrock and Yair Harel from Dr. Hoffman's group, in collaboration with Prof. Wenwei Chang from Arizona State University, set out to answer this question. First, they tested how sensitive disordered proteins are to changes in the intracellular environment. The scientists focused on a network of more than 100 proteins that regulate gene expression in the cell and selected from it five disordered proteins that work together and perform key roles within this protein network. The researchers attached red and green fluorescent tags to the two ends of each protein molecule, put the proteins into a solution and observed them through a microscope, while changing the concentration of salts in the solution, similar to changes in the concentration of salts that occur frequently in living cells. The ratio between the light emitted from the red and green tags indicated to them if and when structural changes occurred in the protein molecules.
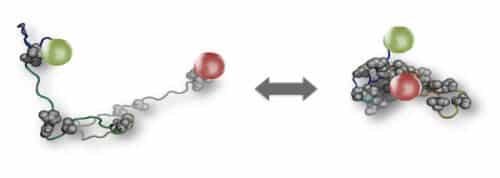
This experiment revealed that all five disordered proteins were sensitive to environmental changes: their three-dimensional structures changed according to changes in the salt concentration in the solution. However, surprisingly, calculations made by the researchers based on the experimental data showed that the changes did not affect the function of the protein network. This is because the five proteins changed in a coordinated manner - they swelled or shrunk together in response to a certain change - so that the overall function of the network remained unchanged. "We showed that disordered proteins are sensitive to their environment," says Dr. Hoffman and adds: "But we also showed that the network they create masks this sensitivity and remains stable enough to continue functioning despite the changes."
These findings indicate that disordered proteins form resistant networks suitable for carrying out essential tasks in the cell. The findings may shed light on cellular processes in which these proteins participate; For example, disordered proteins play key roles in cancer, including the well-known tumor suppressor P53 and the oncogene called c-Myc.
About 50% of the sensitivity of disordered proteins to the intracellular environment is due to the fact that they are disordered.
