The Swedish Academy of Sciences today awarded the 2019 Nobel Prize in Chemistry to three researchers for their contribution to the development of the lithium-ion battery: Bannister Goodenough, Manley Stanley Whittingham and Akira Yoshino. Although this is an invention from the seventies, today there is progress in the field thanks to the need to extend the distance allowed by batteries in electric cars

The Swedish Academy of Sciences today awarded the 2019 Nobel Prize in Chemistry to three researchers for their contribution to the development of the lithium-ion battery: Bannister Goodenough, Manley Stanley Whittingham and Akira Yoshino.
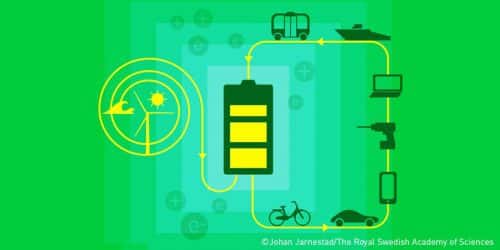
Lithium ion batteries have led to a breakthrough in all aspects of our lives and are used in many areas, from mobile phones to laptops and electric vehicles. Thanks to their research, the winners of the 2019 Nobel Prize in Chemistry laid the foundation for a wireless and fossil fuel-free society. Various companies are currently developing batteries with a huge capacity to allow the storage of energy produced from the sun during the day for use at night to facilitate the transition to renewable energy. In fact, this is a classic award according to the spirit of Alfred Nobel's will - that the prize should be given for inventions that advance humanity.
In the early XNUMXs, Stanley Whittingham, this year's winner of the Nobel Prize in Chemistry, used the energetic power of the lithium atom to release its outermost free electron while developing the first ever functioning lithium battery.
2019 Nobel Laureate in Chemistry John Bannister Goodenough has doubled the potential of the lithium-ion battery, creating the right conditions to get more power from the battery.
The winner of the Nobel Prize in Chemistry for 2019, Akira Yoshino, succeeded in 1981 in replacing the pure and flammable lithium from the battery and instead using lithium ions, which are safer than pure lithium. This replacement allowed the researchers to provide a finished and safe product for industry and everyday life.
The winners of this year's Nobel Prize in Chemistry have developed the most powerful battery in the world
Rarely does an element take center stage in the drama, but the story of the 2019 Nobel Prize in Chemistry has a main character: lithium, an ancient element created during the first minutes of the Big Bang. Mankind became aware of it in 1817, when the Swedish chemists Johan August Arfodsson and Vince Jacob Berzelius isolated it from a mineral sample that came from a mine in Sweden.
Berzelius named the new element after the Greek word for stone, lithos, as lithium. It is the lightest solid element, a fact that explains why today we hardly feel the weight of the mobile phones in our pockets. To be fair, the Swedish chemists didn't actually find pure metallic lithium, but lithium ions in the form of a salt. Pure lithium caused many fire alarms, despite its high level of flammability - it is an unstable element that must be kept in an oil so that it does not react with the air and ignite.
Lithium's weakness - its high reactivity - is also its strength. In the early 1980s Stanley Whittingham used the energetic power of the lithium atom to free its outermost free electron while developing the first ever functioning lithium battery. In 1985, John Goodenough doubled the potential of the lithium-ion battery, creating the right conditions to get more power from the battery. In XNUMX, Akira Yoshino managed to replace the pure and flammable lithium from the battery and instead use lithium ions, which are safer than pure lithium. This replacement allowed the researchers to provide a finished and safe product for industry and everyday life. Lithium-ion batteries have provided mankind with the greatest benefit of all, in that they allow the development of portable computers, mobile phones, electric vehicles and allow the storage of energy generated by solar and wind power.
Now, let's go back fifty years to the beginning of the story of the lithium ion battery.
Gasoline fumes are reviving battery research
In the middle of the XNUMXth century, the number of cars powered by gasoline increased significantly, and the exhaust fumes caused by their driving on the streets worsened the condition of the harmful smog that enveloped large cities. This situation, combined with the growing recognition among the general public that oil and its products are a depleting source of energy, sounded the alarm for both vehicle manufacturers and oil companies. They were required to invest in electric vehicles and alternative energy sources if they wanted to continue to survive.
Electric vehicles and alternative energy sources both require powerful batteries that can store large amounts of energy. At that time there were only two types of rechargeable batteries on the market: the heavy lead battery that was invented back in 1859 (and which is still used as a starting battery in gasoline-powered vehicles) and the nickel-cadmium battery that was developed in the first half of the twentieth century.
Oil companies invest in new technology
The fear of the oil in the ground led the giant oil company Exxon to decide to diversify its activities. As part of its significant investment in basic research, the company recruited a number of researchers who were the leaders in their field at the time in the field of energy, and gave them the freedom to research as much as they wanted, as long as their research did not deal with oil.
Researcher Stanley Whittingham was one of those researchers who came to Exxon in 1972. He came from Stanford University, where he studied solid materials with atomic-sized voids into which ions can bind. This phenomenon is called intercalation. The properties of the material change when ions are trapped within them. At Exxon, Stanley Whittingham and his colleagues began researching superconducting materials, including tantalum disulfide, which can trap ions within it. They added ions to the material and studied how this affected the electrical conductivity of the material.
Whittingham discovers a particularly high-energy substance
As happens in science, this experiment led to an unexpected and valuable discovery. It turned out that potassium ions affected the conductivity of tantalum disulfide, and when Stanley Whittingham began to study the material in detail he noticed that it had a very high energy density. The interactions between the potassium ions and the tantalum disulfide were, surprisingly, high in energy, and when he measured the voltage of the material, the value was several volts. This result was better than many of the batteries available at the time. Stanley soon realized it was time to change course and began developing new technology that could store energy for the electric vehicles of the future. However, tantalum is one of the heavier elements and the commercial market did not need additional heavy batteries - so he replaced tantalum with titanium, an element similar in its properties but lighter in weight.
Lithium in the negative electrode
The choice of lithium was not a random choice; In a battery, electrons are supposed to flow from the negative electrode - the anode - to the positive electrode - the cathode. Therefore, the anode should contain a material that easily gives up its electrons, and of all the elements known to us, lithium is the element that gives up its electrons most easily.
The result was a rechargeable lithium battery that operated at room temperature with tremendous potential. Stanley Whittingham traveled to Exxon headquarters in New York to present his venture. The meeting lasted fifteen minutes and the board member came to a quick decision: they would develop a commercial battery based on Whittingham's discovery.
The first rechargeable batteries contained solid materials in the electrodes, which decomposed in a chemical reaction with the electrolyte. This situation destroyed the battery. The advantage of Whittingham's lithium battery lay in the fact that the lithium ions were stored in the spaces inside the titanium disulfide that made up the cathode. When a battery is used, lithium ions move from the lithium in the anode to the titanium disulfide in the cathode. When the battery is charging, the lithium ions move in the opposite direction.
The battery explodes and the price of oil plummets
Unfortunately, the research group preparing to manufacture the battery experienced a number of bumps. As the lithium battery underwent repeated charging, a deposit layer of lithium formed on the lithium electrode. When particles of this layer reached the counter electrode, the battery experienced a short circuit that could have led to an explosion. The fire department had to extinguish several fires and in the end they threatened to make the laboratory pay for the special chemicals used to extinguish lithium fires.
In order to make the battery safer, the researchers added aluminum to the metallic lithium electrode and replaced the electrolyte between the electrodes. Stanley Whittingham announced his discovery in 1976 and the company began producing this battery on a small scale for a Swiss watchmaker who wanted to use it in solar powered watches.
The next goal was to increase the production volume of the rechargeable lithium battery so that it could power a car. However, the price of oil dropped dramatically in the early XNUMXs and Exxon had to make cuts. Development work was frozen and the patent on Whittingham's battery technology was transferred to the ownership of three companies located in three different parts of the world. However, this situation did not lead to the halt of development. When Exxon gave up, the development work was continued by researcher Goodenough.
The oil crisis makes Goodenough interested in batteries
As a child, John Goodenough experienced serious problems in learning to read, one fact that led him to study mathematics and eventually - after World War II - to study physics as well. He worked for many years at Lincoln Laboratory at the Massachusetts Institute of Technology (MIT). While working there, he contributed to the development of the technology of random access memory (RAM) which is still a basic component of computers today.
John Goodenough, like many people in the seventies, was affected by the oil crisis and wanted to contribute to the development of alternative energy sources. However, Lincoln Laboratory was funded by the US Air Force which did not allow any kind of research, and so when he was offered a position as a professor of inorganic chemistry at the University of Oxford in Great Britain, he took advantage of the opportunity and entered the important field of energy research.
High voltage is obtained when lithium ions are hidden inside cobalt oxide
John Goodenough was familiar with Whittingham's revolutionary battery, but his in-depth knowledge of the inner material of the electrodes led him to conclude that he could achieve a higher potential if he replaced the metal sulfide with a metal oxide. After that, several researchers in his research group were tasked with finding a metal oxide that would generate high voltage when it reacted with lithium ions, but one that would not break down when the ions were removed from it.
This systematic study was much more successful than John Goodenough dreamed of. Whittingham's battery produced more than two volts, but Goodenough found that the battery containing lithium-cobalt oxide at the cathode was almost twice as powerful, producing a value of four volts.
One of the reasons for this success was John Goodenough's understanding that the batteries do not have to be manufactured in their charged state, as was customary in the past. Instead, they could be recharged afterwards. In 1980, he published the discovery of this new, high-energy cathode material that was also light-weight, leading to the development of powerful batteries with high energy capacity. It was a clear step towards the wireless revolution.
Japanese companies are developing lightweight batteries for new electronic components
However, in the West, as oil became cheaper, interest in investing in alternative energy technology and the development of electric vehicles grew. Things were different in Japan - electronics companies there were already desperate in their search for rechargeable, lightweight batteries that could power innovative electronic components, such as video cameras, cordless phones and computers. One of the people who became aware of this need was Akira Yoshino of Asahi Kasei. Or as he put it: "I simply sniffed the direction in which the trends were headed. You could say I had a good sense of smell."
Yoshino assembles the first commercial lithium-ion battery
When Akira Yoshino decided to develop a functional rechargeable battery, he was familiar with Goodenough's cathode composed of lithium-cobalt oxide, and thus he tried using several carbon-based materials as the anode. The researchers already knew that lithium ions could be integrated between the molecular layers of graphite, but the graphite disintegrated in contact with the electrolyte in the battery. Akira Yoshino's revolutionary idea came when he tried, instead, to use petroleum coke, a byproduct of the oil industry. When he charged the petroleum coke with electrons, the lithium ions were drawn into the material. Next, when he turned on the battery, the electrons and lithium ions flowed toward the cobalt oxide in the cathode, which had a higher potential.
The battery developed by Akira Yoshino is stable, lightweight, high capacity and produces a high voltage of four volts. The biggest advantage of the lithium-ion battery is that the ions are integrated within the electrodes. Most other batteries are based on chemical reactions, following which the electrodes change with certainty, even if at a slow rate. When charging or using a lithium ion battery, the ions flow between the electrodes without reacting with their environment. This means that the battery has a long life and can be charged hundreds of times before its performance becomes poor.
Another big advantage lies in the fact that the battery does not contain pure lithium. In 1986, when Akira Yoshino tested the safety of the battery, he took the precaution of using a special device designed to test devices with the potential to explode. He dropped a large piece of iron on top of the battery, but nothing happened. However, repeating the experiment with a battery that contained pure lithium resulted in a serious explosion.
The battery's success in passing the safety test was crucial to the battery's future. Akira Yoshino says this was "the moment when the lithium-ion battery was born".
The lithium-ion battery is essential for a fossil fuel-free society
In 1991, a large Japanese electronics company began selling the first lithium-ion batteries, revolutionizing the world of electronics. Mobile phones shrank in size, computers became portable and music players and tablet computers were developed for the first time.
As a result, researchers around the world scanned the periodic table of the elements in order to develop even more efficient batteries, but none of them has yet succeeded in inventing a more efficient system than the lithium-ion battery in terms of capacity and voltage it produces. However, the lithium-ion battery has been modified and improved: among other improvements, researcher John Goodenough replaced the cobalt oxide with iron-phosphorus, a replacement that makes the battery more environmentally friendly.
Like everything else, the production of lithium-ion batteries also has a harmful effect on the environment, but these batteries also have many environmental benefits. The batteries enabled the development of cleaner energy technologies and the development of electric vehicles, and therefore they contributed to reducing the emission of greenhouse gases and harmful particles.
Thanks to their work, researchers John Goodenough, Stanley Whittingham and Akira Yoshino created the right conditions for a wireless and fossil fuel-free society, thus making the greatest contribution to humanity.
More of the topic in Hayadan:
Next-generation lithium batteries: longer duration of activity, less charging time
An innovative material that could create a breakthrough in hydrogen vehicles
Build better batteries
Israel is testing wireless charging of electric vehicles
