A new sponge-like material, crisp and black in color, prepared by freeze-drying is capable of performing quite impressive tasks
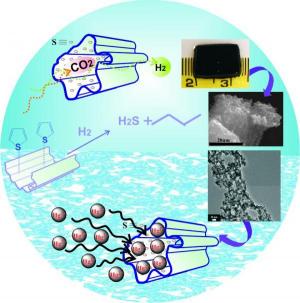
A new sponge-like material, crisp and black in color, prepared by freeze-drying (similar to the ice cream that the astronauts eat) is capable of performing quite impressive tasks. The material, prepared by chemists from Northwestern University, is able to remove mercury from polluted water sources, separate hydrogen from other gases and is a more effective catalyst than the one currently used to remove sulfur compounds from crude oil.
Hydrodesulfurization is a very common catalytic chemical process that removes sulfur from natural gas and petroleum distillates, such as gasoline, diesel and jet fuel. In the absence of this efficient process, we would continue to burn sulfur - a process that contributes to the formation of acid rain.
Scientists have tried to improve the process, but without success. Many of the scientists believe that the process that exists today is the best possible. The university researchers, in collaboration with their colleagues at Western Washington University, report that their new material is twice as active as the currently common catalyst for the process, while consisting of the same components.
The new material, cobalt-molybdenum-sulphur, is a new type of chalcogels, a family of materials that was discovered only a few years ago at the same university. These materials are irregular networks of metal-sulfur atoms with a very wide surface area. The new material consists of common elements, is stable when exposed to air or water and can be obtained in powder form.
Details about the new material and its properties were published in the scientific journal Nature Chemistry. This is the first article regarding the use of such materials for chemical catalysis and gas separation.
"I was surprised by the impressive activity of our catalyst, given the difficulties the researchers faced in their previous attempts to improve the removal process of the right sulfur," said the paper's lead author, chemist Mercouri G. Kanatzidis. "Basically, our catalyst will be able to operate on twice the amount of crude oil than the existing catalyst. These days we are conducting tests to examine how the catalyst works under more industrial conditions."
The researchers prepared their catalyst using a method different from the usual one. The material is a gel composed of cobalt, nickel, molybdenum and sulfur, which in the next step undergoes a freeze-drying process to obtain a sponge-like material with an extremely large surface area - in the volume of one cmXNUMX of the material, there is a surface area equal to about half the area of a football field. This extensive surface area and the stability of the material under catalytic conditions are what make it an active varnish.
The researchers also showed that the material is capable of absorbing toxic heavy metals from polluted water sources with unprecedented efficiency, which does not exist in any other known material. The material was able to remove about ninety-nine percent of the mercury found in polluted water at a concentration of only a few parts per million. Mercury tends to bind to sulfur atoms, which are present in large quantities in the new material.
Two years ago, the same researchers reported on an alcohol gel capable of removing mercury from a liquid, but this substance contained the precious substance platinum; The state-of-the-art chalcogel contains only precious elements, with nickel and cobalt replacing the platinum. The nickel and cobalt atoms bind to the sulfur atoms of the thiomolybdate anions to form a porous three-dimensional structure.
"We were able to perform a particularly difficult task: to completely replace the platinum using only common materials to create the gel," explains the researcher. "We found the right conditions to get the features we need. The key ingredient was changing the solvent from water to formamide.”
In addition to being a better catalyst for removing sulfur compounds and bonded mercury, the material is also very effective for gas separation. The researchers showed that the material easily removes carbon dioxide from hydrogen - an application that could be useful in the hydrogen industry. The gas separation process utilizes the "soft" sulfur atoms found in high concentration on the surface of the new material. These atoms tend to react with other soft particles that pass by them so that their rate of movement slows down in this passage. Hydrogen, the smallest element, is a "hard" element. The hydrogen simply "slides" through the material while soft particles such as carbon dioxide stay in it longer.

2 תגובות
Where do you buy one? My Tami4 is already sleeping.
Does he also know how to make coffee?