Prof. Uri Benin from the Institute of Chemistry and the Center for Nanoscience and Nanotechnology succeeded for the first time in combining two quantum dots to create stable artificial molecules, and now he believes that the discovery will affect technology in the future: "One can imagine the exciting possibilities for creating a selection of artificial molecules, with the promise of using them in technological applications many"
In 1869, Dmitri Mendeleev began to classify the elements according to their chemical properties, and invented a table that would later be called the "Periodic Table". "I saw in a dream a table in which all the elements fell into place as required. I woke up and immediately wrote it down on a piece of paper," Mendeleev told how he created the first periodic table. Since then, more elements have been discovered and with their help new molecules have been created, as scientists now know about 150 million molecules that consist of the 118 atoms of the elements of the periodic table. 150 years after Mendeleev's discovery, Prof. Uri Benin and his team from the Institute of Chemistry and the Center for Nanoscience and Nanotechnology at the Hebrew University created new molecules made of artificial "atoms", known as quantum dots. The research findings were published in the latest edition of Nature Communications.
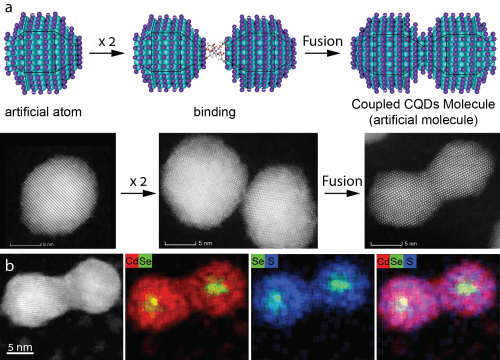
Prof. Benin and his team succeeded for the first time in connecting two quantum dots and created a new molecule. Quantum dots are nanometer-sized crystals, each containing hundreds to thousands of semiconductor atoms. The quantum dots are called 'artificial atoms' due to the fact that many of their properties resemble properties of the atoms we know from the periodic table. When these artificial atoms are combined together, they create a new artificial molecule with unique properties and characteristics, similar to making a molecule from atoms.
It is worth noting that this is not the first time in history that researchers have tried to connect quantum dots. In the past, they managed to create a thin connection between quantum dots, a sort of "bridge", which did not last long. All their efforts to create a stronger bond were in vain. Prof. Benin and his team were able to connect the two quantum dots together until a unified crystal was formed, thus creating a bond between them that turned the two quantum dots into a stable molecule that also shows coupling phenomena that characterize normal molecules.
At the Hebrew University, Prof. Benin's colleagues define his scientific work as "a real breakthrough". According to one of them, the research carried out by Prof. Benin is an "initial step" to "create a real molecule from quantum dots and not something improvised, as was known until today. So far, researchers around the world have succeeded in creating a kind of 'rope' that connects two quantum dots and nothing more than that." In future studies, Prof. Benin will strive to create artificial molecules from quantum dots with diverse compositions. That is, a sort of periodic table of artificial atoms will be created to create new nanomolecules.
Unlike the known atoms in the periodic table, the physical, electronic and optical properties of the quantum dots can be changed by controlling their nanometer size. For example, a larger quantum dot will emit red light, while a smaller dot, of the same material, will emit green light. In connection with this, Prof. Benin and his team developed a method by which it is possible to create artificial quantum molecules that are controlled through the design of the artificial atoms that make them up, thereby adjusting the properties of the molecules (for example, the color of the emitted light).
Over the past twenty years, researchers around the world have been able to achieve optimal control over the composition and size of quantum dots and the understanding of the physical properties of these tiny particles has deepened. Indeed, the quantum dots have already become an integral part of our daily life. For example, in solar energy applications and the new generation TV screens, where the quantum dots provide an image with sharp and vivid colors in combination with energy saving. "Given the rich selection of size- and composition-controlled quantum dots, we can only imagine the exciting possibilities for creating a variety of artificial molecules from these artificial atoms. There is a significant potential for their use in many technological applications, such as opto-electronic technologies, sensing technologies, and quantum technologies", Prof. Benin concludes.

7 תגובות
Another PDF version of the article
https://arxiv.org/ftp/arxiv/papers/1905/1905.06065.pdf
It's amazing what you can achieve from Torah studies!
Poppy
Well done ???
Well done!
Why is a quantum dot defined as an artificial atom and not as a large molecule or nanocrystal? Does it behave more like an atom than a molecule? What are its physical and even chemical properties beyond emitting light at different wavelengths?
Very nice
Presidential building!!!!