Mendeleev's ambition led him to discover the periodic laws and he created the periodic table - one of the most iconic symbols in science - almost everyone recognizes it immediately. Very few scientific works receive such recognition. Using the periodic table, Mendeleev predicted the existence and properties of new chemical elements. When these foundations were discovered, his place in the history of science was assured.

Dmitri Ivanovich Mendeleev (1834-1907)
Dmitri Mendeleev was keen on chemistry. His biggest dream was to find a better way to organize the field.
Dmitri Ivanovich Mendeleev was born on February 8, 1834 in Varakhin Armaziani in the Siberian province of Russia. His family was unusually large. He had 16 brothers and sisters, although the exact number is unknown.
His father was a teacher, a graduate of the Teacher Training Institute in St. Petersburg. When his father went blind, his mother reopened a glass factory that the family had opened and closed. His father died when Mendeleev was 13 and the glass factory burned down when he was 15.
At the age of 16, Mendeleev moved with his wife to St. Petersburg, which was then the capital of Russia. He was accepted to the same institution where his father studied and was trained as a teacher. At the age of 20, Mendeleev showed his promise when he published original research papers. He suffered from tuberculosis and often worked from his bed. He graduated from teacher training school as an honors student despite the fact that his uncontrollable temper made him unpopular with his teachers and fellow students.
In 1855, at the age of 21, Mendeleev was hired as a science teacher in Simferopol on the Crimean peninsula but soon returned to St. Petersburg, where he began studying for a master's degree in chemistry at the University of St. Petersburg. He received his degree already in 1856.
chemistry
Trained as both a teacher and an academic chemist, Mendeleev spent most of his time in both disciplines before he was awarded a scholarship that allowed him to conduct chemical research in Western Europe. He spent 1859 and most of 1860 in Heildberg, Germany where he was fortunate enough to work briefly with Robert Bunsen at the University of Heildberg. In 1860 Bunsen and his colleague Gustav Kirchhoff discovered the element cesium using a chemical spectroscope - a method they developed themselves and which Bunsen also presented to Mendeleev. In 1860, Mendeleev also participated in his first international conference in the field of chemistry in Karlsruhe, Germany. Most of the discussions at the conference dealt with the need to standardize chemistry. This convention played a key role in the development of the periodic table of the elements. The table was based on atomic weight and he witnessed the agreement to reach a standard method for determining these weights. At this gathering, he also learned about Avogadro's law which states that "all gases of the same volume, temperature and pressure contain the same number of molecules".
Upon his return to St. Petersburg in 1861 to teach at the Institute of Technology, Mendeleev became even more passionate about the science of chemistry. He also feared that chemistry in Russia was lagging behind the scientific level he experienced in Germany. He believed that improving Russian language chemistry textbooks was necessary and he was determined to do something about it. He worked like a demon. Within 61 days, the 27-year-old poured all his knowledge of chemistry into a 500-page textbook - "Organic Chemistry". This book won the Domidov Prize and placed Mendeleev at the forefront of chemical education in Russia.
Mendeleev was a charismatic teacher and lecturer and held several academic positions until in 1867 - when he was only 33 years old - he won the position of head of the department of general chemistry at the University of Saint Petersburg.
In this prestigious position he decided to push forward and improve chemistry in Russia. He published the principles of chemistry in 1869. Not only did this textbook become popular in Russia, it was popular in other parts of the world when it was translated into English, French and German.
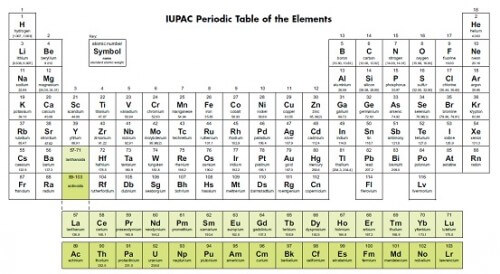
the periodic table
In that chemical period there was a patchwork of observations and discoveries. Mendeleev was sure that there were more fundamental principles. This idea remained in the background when he began in 1869 to write the second volume of his book Principles of Chemistry.
At the heart of chemistry were the elements. "What can they reveal to us if we find ways to organize them in a logical way"? Ask.
He wrote the names of the 65 elements known at that time on cards, like playing cards - one element on each card. He then wrote the basic properties of each element on that card, including its atomic weight. He saw that atomic weight was important because it affected the behavior of the elements that seemed to repeat itself as atomic weight increased - but he didn't see the pattern yet.
He was convinced he had discovered something disciplinary. He moved the cards from place to place and changed the order until he finally fell asleep on the desk. When he woke up he found that the subconscious had done the work for him. Now he knew the pattern that elements followed. He later wrote: "In my dream I saw a table where all the elements fall into their designated place. When I woke up I immediately wrote it down on paper."
It took him several weeks to publish the relationship between the atomic weights of elements and the properties of matter. The periodic table has already been released to the scientific world.
Why was Mendeleev's periodic table successful?
Like many discoveries in science, there is a time when an idea becomes ripe for discovery, and this was the case with the periodic table in 1869. Lowther Meyer, for example, proposed a rough periodic table in 1864. In 1868, another chemist came up with a table that was very similar to Mendeleev's, but he He did not publish it until 1870.
John Newlands published a periodic table in 1865. Newlands recognized the periodic behavior of the elements and wrote: "Any given element will show a behavior similar to the eighth element following it in the same table" Newlands also predicted the existence of a new element (germanium) based on a gap in his table. Unfortunately for Newlands, his work was ignored.
The reason Mendeleev became the leader of the group was probably because he not only showed how the elements could be organized but also predicted the behavior of yet-to-be-discovered elements. He claimed that the reason why some of the elements whose behavior did not match his predictions was because their atomic weights were measured incorrectly. Mendeleev predicted the existence of eight new elements and predicted their properties. It turns out that when chemists checked the atomic weights it turned out that the previous measurements were wrong and Mendeleev was right. Now chemists all over the world have started using the table.
As new elements suitable for his prediction were discovered, Mendeleev's reputation for scientific fame rose. In 1905, the British Royal Society gave him its highest honor, the Copley Medal, and in the same year he was elected to the Royal Swedish Academy of Sciences.
The 101st element is named Mandelbium in his honor.
More of the topic in Hayadan:

9 תגובות
hi father
In my opinion, the background story is a bit lacking, I think...
His father died, the brothers were "released" by the mother who took Mendeleev to Moscow University where they did not accept him, so from there they rode to St. Pat.. that's where he was accepted for studies, the factory burned down about a year after his father's death.. and some other changes in relation to what you wrote down,
Of course he was not the only one who worked in the field, but his arrangement was groundbreaking.
Of course, they did not discover the eighth column of the nobles - at that time they had not yet been discovered - in any case, an amazing story!
In addition, it is important
joins This may harm the promotion of the site. And word's spell check (press F7) will fix the problem.
Thanks for the comment. I'll take care of it in the morning.
my father
An interesting and good article, but why is it littered with proofreading mistakes?! Shouldn't it have been checked a moment before publication?
Steve
The basis of modern science is that there is order. The mickey is not popular among scientists, and it is known that Einstein, for example, was very disturbed by it.
With us, even those who don't sleep remain "smart at night". Fact. See where they roll us.
He simply decided to organize the existing knowledge and by chance he noticed that there was a systematic repetition. I have no idea about the extent of his religiosity, but not every order in nature is a product of creation, there are feedbacks and other methods that cause order.
An impressive review of an impressive person
Peace and blessings, my father.
Why did Mendeleev even assume that there is some order in nature? Did he not believe in accidental creation?