The 2016 Nobel laureate discovered and deciphered the mechanisms underlying the process of autophagy (or autophagocytosis), a fundamental process for the breakdown and recycling of cellular components

The Karolinska Institute in Sweden announced the winner of the 2016 Nobel Prize in Medicine/Physiology - Prof. Yoshinori Osumi from Japan for the discovery of the "autophagy" mechanism.
The 2016 Nobel laureate discovered and deciphered the mechanisms underlying the process of autophagy (or autophagocytosis), a fundamental process for the breakdown and recycling of cellular components.
The term autophagy originates from the Greek words "auto-", which means "self", and the word "pegian", which means "to eat", that is - "self-eating". The idea began to grow in the sixties of the last century, when researchers discovered for the first time that the cell is capable of destroying its own internal contents by dissolving them in membranes, creating sac-like vesicles that were transferred into a biological system dedicated to breaking down biological substances called a lysosome. Difficulties in studying the mechanism led to extremely limited knowledge in this field, until the early nineties, when, in a series of brilliant experiments, the researcher Yoshinori Osumi used yeast cells to identify genes essential to the functioning of the mechanism. In the next step, he was able to decipher the mechanisms underlying the process in yeast and proved that similar complex mechanisms also work in our cells.
These discoveries led to a new paradigm in our understanding of the mechanism by which the cell recycles its contents. His discoveries paved the way for understanding the fundamental importance of autophagy in many physiological processes, such as the adaptation to starvation conditions or the response to infection. Mutations in the genes responsible for the process can cause diseases, and in addition, the process itself is involved in certain diseases such as cancer and neurological diseases.
Decomposition - a central process in all living cells
In the mid-fifties of the last century, researchers noticed that a new specialized cell component, called an organelle, contains enzymes that break down proteins, carbohydrates and fats. The designated component is called "lysosome", and it functions as a factory for breaking down cellular components. The Belgian researcher Christian de Doub was awarded the Nobel Prize in Physiology in 1974 for the discovery of the lysosome. Additional observations in the sixties showed that a large amount of cellular content, and even whole organelles, can sometimes be found inside the lysosomes. That is - the cell has the possibility to transfer a large amount of biological material into the lysosome. Further biochemical and microscopic research showed that there is a new type of mechanism for transferring cellular material in the form of vesicles into the lysosome for its degradation (Figure 1). Christian de Doub, the researcher responsible for the discovery of the lysosome, called the process autophagy. The new organelles were called autophagosomes.
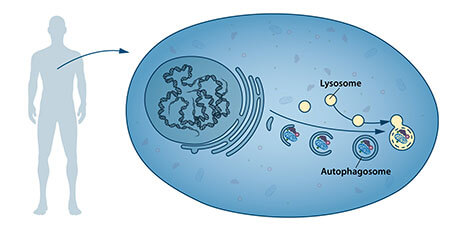
During the seventies and eighties, researchers focused on deciphering another system used to break down proteins called the proteasome. As part of this field of research, the three researchers Aharon Chechenover, Avraham Hershko and Irwin Rose won the 2004 Nobel Prize in Chemistry for "the discovery of protein degradation mediated by ubiquitin". The proteasome manages to successfully break down proteins, one by one, however, this mechanism could not explain how the cell removes larger protein aggregates and even whole organelles that have ended their lives.
A groundbreaking experiment
The researcher Yoshinori Osumi was involved in a variety of research fields, but when he established his own laboratory in 1988, he focused on the mechanism responsible for breaking down proteins in the vacuole, an organelle in plant cells that is functionally similar to the lysosome organelle in human cells. Yeast cells are relatively easy to study, so they are often used as a model for human cells. They are particularly useful in locating genes important in complex cellular pathways.
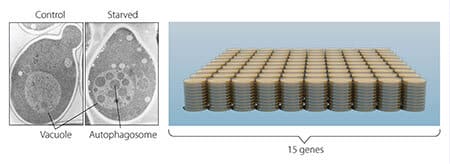
However, the researcher encountered a difficult challenge - yeast cells are small cells and therefore it is not possible to easily differentiate between the various internal structures within them under a microscope. The researcher assumed that if he disrupted the degradation process in the tube while the autophagy process was still ongoing, then autophagosomes should accumulate inside the tube and allow them to be viewed with a microscope. Therefore, he cultured mutant yeast cells from which the degradation enzymes in the capsule were removed and at the same time accelerated the autophagy process by starving the cells.
The results were amazing - within a few hours the cavities were filled with small organelles that did not undergo decomposition (Figure 2). These organelles were the autophagosomes and this experiment proved that the autophagy process does occur in yeast cells. These results constituted an impressive scientific breakthrough when the researcher published them in 1992.
The discovery of the genes responsible for the autophagy process
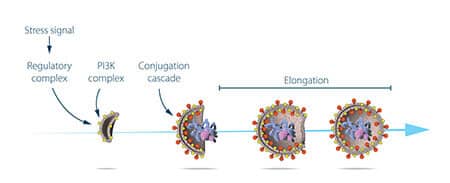
At this stage, the researcher used the genetically engineered yeast strains in which autophagosomes accumulated following starvation. This accumulation should not occur if the genes essential for the autophagy process are disabled. The researcher exposed the yeast cells to a chemical that causes random mutations in many of the genes, and then he induced autophagy. His strategy was successful - within a year of the discovery of the autophagy process in yeast, Osumi identified the first genes essential to the process. In a series of successive experiments, he was able to functionally characterize the proteins encoded by these genes. The results demonstrated that the autophagy process is controlled by a series of proteins and protein conjugates, each of which regulates a distinct and defined step in the initiation and progression of autophagosomes (Figure 3).
Autophagy - an essential mechanism in our cells
After identifying the mechanism of the autophagy process in yeast cells, a key question remains - is there a corresponding mechanism in other organisms? It soon became clear that almost identical mechanisms exist in our own cells. The research tools required to study the importance of the autophagy process in humans were already available to researchers.
Thanks to Osumi and other researchers who followed him, we now know that the autophagy process is responsible for important physiological functions where cellular components are required for breakdown and recycling. Autophagy can rapidly provide fuel and building blocks required for the renewal of cellular components, and is therefore essential for the cellular response to starvation and other types of stress. After the development of an infection, the autophagy process can remove viruses and bacteria that have invaded the cells. The cells also use the autophagy process to mitigate the negative consequences of the aging process.
Disruption of the autophagy process has been linked to Parkinson's disease, type 2 diabetes and other diseases that appear in old age. Mutations in the genes responsible for the process can lead to genetic diseases. Disruption of autophagy has also been linked to cancer development. Today, vigorous research is being conducted with the aim of developing drugs capable of affecting the autophagy process in a variety of diseases.
The process of autophagy has been known for fifty years, but its fundamental importance in the fields of physiology and medicine was only recognized after the groundbreaking research of Prof. Yoshinori Osumi in the nineties. For these discoveries, the researcher wins the Nobel Prize for this year in the field of physiology/medicine.
Yoshinori Osumi was born in 1945 in Fukuoka Prefecture, Japan. He received his doctorate from the University of Tokyo in 1974. After three years at the Rockefeller University in New York, USA, he returned to the University of Tokyo where he established his research group in 1988. Since 2009, he has been a professor at the Tokyo Institute of Technology.

One response
what is this shit??? The information here is not useful for sailing