Scientists from UCLA University in California and Korea University have developed an innovative method that allows nanomachines to release drugs inside living cancer cells after being remotely activated by a magnetic field.
Scientists from UCLA University in California and Korea University have developed an innovative method that allows nanomachines to release drugs inside living cancer cells after being remotely activated by a magnetic field.
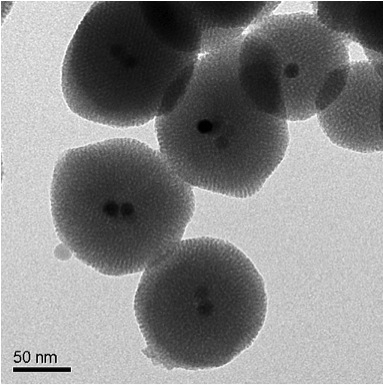
The new method - which for the first time ever utilizes porous nanomaterials containing a magnetic core - has the ability to improve both directed drug release and magnetic resonance imaging in the treatment of cancer and other diseases. The research findings were published in the scientific journal Journal of the American Chemical Society.
In recent years, cancer research has focused on the development of healing methods, which, unlike chemotherapy, can act selectively only on the cancerous cells while avoiding damage to the healthy cells as well. To date, scientists have prepared nanomachines that are capable of storing drug particles in their contents and releasing them when needed through pores directly into the interior of individual cancer cells in response to an external signal.
While many methods have been developed to control the timing of the opening and closing of the pores and the release of the charge through them for medical applications, an external and non-invasive initiation method is the most preferred to achieve the most effective and safest results.
The new method, developed by research groups led by Jeffrey Zink, professor of chemistry and biochemistry at UCLA, and researcher Jinwoo Cheon, professor of chemistry at Korea University, uses a material that includes a combination of a network of porous silica nanoparticles, together with magnetic nanocrystals of iron oxide that have undergone Doping with zinc, and in addition to them nano-valves that help keep the drug particles inside the pores. When this material is exposed to a signal from a magnetic field, the valves open and release the drug particles from the pores into the target cells.
"The hydrophobic nature of the interior of the pore walls, as well as the ability to change the surface of the silica surface by adding hydrophilic groups, make these particles a preferred material for releasing anticancer drugs," said the lead researcher. "Adding a magnetic core to the silica-based nanoparticles is an interesting capability due to its possible applications in magnetic resonance imaging as well as the use of this magnetic core as a contrast agent."
To test the effectiveness of the new method, nanoparticles loaded with the anti-cancer drug doxorubicin were injected into breast cancer cells. When the cells were exposed to a magnetic field, the cells began to die.
"The silica nanoparticles containing a magnetic core are effective in initiating the nanovalves, which release anti-cancer drugs upon exposure to a magnetic field," the researcher noted.
The activation of the magnetic field causes the magnetic nanocrystals of the iron oxide doped with zinc to heat up. This increased heating causes the molecular machines to start their activity, and thus the medicine in the pores is released out into the cells.
"Magnetic nanocrystals are important in biomedical applications since they can be used both in healing and in imaging and preliminary diagnosis," said the researcher. "The ability to direct anti-cancer drugs to the cancer cells only without harming the healthy cells next to them is an important factor," notes the researcher.
The next step in the research will be to examine the effects of the innovative method in the living body and determine if it can be used to selectively control the location and timing of the release of the drugs. The ultimate goal will be to develop this system to treat cancer patients.
