Prof. Mishna Galia Mein of the Shulich Faculty of Chemistry at the Technion has developed an innovative catalyst that significantly improves the production process of hydrogen from water

Decomposing water to create hydrogen is a challenge that has occupied many research groups around the world in recent decades. This is because hydrogen fuel is a green and ecological substitute for existing fuels: it is produced from a cheap and available resource - water - and the by-product of a hydrogen-powered vehicle is also water, in contrast to the polluting smoke emitted by gasoline-powered vehicles.
In an article published in the journal Nature Catalysis Associate Professor Galia Ma'in of the Shulich Faculty of Chemistry at the Technion presents a molecular complex, or an artificial cluster of molecules, which dramatically improves the efficiency of water decomposition by electricity. This is through biomimetics - an engineering field based on the inspiration of nature (bio - life, mimetic - imitation). In this specific case, it is inspired by the processes of photosynthesis in nature.
Photosynthesis is a natural process that developed during evolution in plants, bacteria and algae. In this process, solar energy is used to convert water and carbon dioxide into organic matter and oxygen. This process is essential for life on Earth, since all animals that are unable to carry out photosynthesis, including humans, feed on the food chain that begins with the photosynthetic bacteria. Furthermore, the oxygen we breathe also originates from photosynthesis.
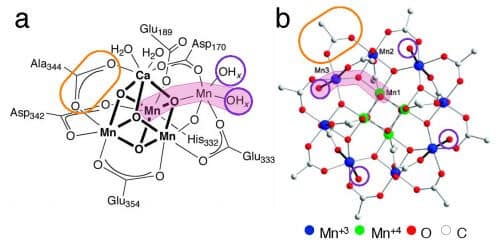
One of the essential elements in the process of photosynthesis is manganese, whose chemical symbol is Mn. Inspired by nature, many attempts have been made to mobilize manganese as a catalyst in breaking down water for the production of hydrogen - a process called electrolysis of water. The water molecule H2O contains an oxygen atom and two hydrogen atoms, and its decomposition is carried out by the flow of electrical energy. This is through a cathode and an anode - the cathode recycles the water, meaning it donates electrons to it and attracts the oxygen to it; And the anode takes electrons from them and attracts the hydrogen to it. The challenges in this process are many, as in many cases an investment of a lot of energy is required to start the process. Furthermore, many catalysts are destroyed in this process and lead to its end.
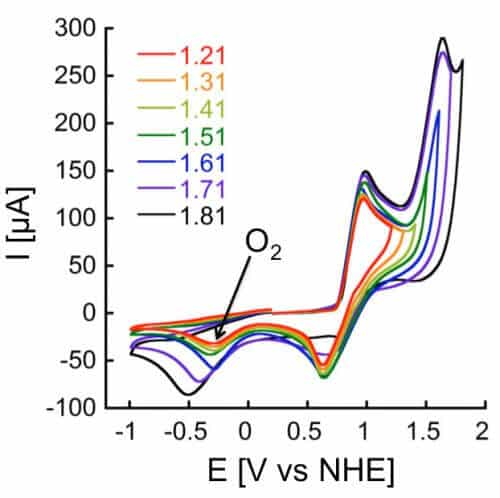
The molecular complex developed by Prof. Mishna Mein is expected to change the situation. to the aforementioned complex, which is actually a complex molecule called Mn12DH, unique properties that give it many advantages in breaking down water. In the experiments done in this complex it was found that it produces a large amount of electrons (electric current) and a significant amount of oxygen and hydrogen, and this with a relatively low energy investment. Equally important, it is stable - that is, it is not destroyed quickly as is the case with other molecules used as a catalyst. According to Prof. Mein, "in nature, evolution has created a protein shell around manganese that stabilizes it and prevents its decomposition. Inspired by the natural structure, we developed an organic shell that allows the solubility of Complexex, which plays in water, and stabilizes it."
Most of the work described in the article was done by the student Naama Glauz as part of her master's degree studies under the guidance of Associate Professor Ma'in. Glauz continues her research on the unique Mangan complex as part of her doctoral studies. In preliminary studies she was able to show that the complex is able to break down water by exposure to light from a simple lamp. In this way, it will be possible to produce oxygen and hydrogen in large quantities very quickly, and the future idea is to rely on solar energy in this process without needing electricity.
Associate professor Mein fell in love with the field of chemistry in the first chemistry class in the 2012th grade "and I knew right away that I wanted to be a chemistry professor." After the army, she completed a bachelor's degree in chemistry at Tel Aviv University (with honors), a master's and a third degree at the Weizmann Institute, and a post-doctorate at New York University (NYU) and the University of Florida - where she began researching the manganese complex. Since XNUMX, she has been the head of the biomimetic chemistry laboratory at the Shulich Faculty of Chemistry at the Technion.
See more on the subject on the science website:

2 תגובות
A liter of water = 1000 km. How do you take tax? At least small power plants at the level of a washing machine. It won't be taking the cherry on top of the lion... and then maybe a poor look will materialize. Engines that run on 70 percent water.!!!!!
If it's as good as it says in the article, good luck!
Hope to see in a few years cars running on hydrogen technology on Israel's roads, and even trains, buses and trucks.