Engineers at the University of Wisconsin-Milwaukee have discovered a catalyst capable of yielding the same level of efficiency in microbial fuel cell applications as the currently used platinum catalyst and at five percent of its cost.
Engineers at the University of Wisconsin-Milwaukee have discovered a catalyst capable of yielding the same level of efficiency in microbial fuel cell applications as the currently used platinum catalyst and at five percent of its cost.
Given the fact that more than sixty percent of the financial investment in the development of microbial fuel cells is related to the cost of the platinum metal, the current discovery can lead to the development of more efficient devices for converting and storing energy.
The material - iron-carbon nanorods rich in nitrogen atoms - also has the ability to replace the platinum catalyst used today in microbial electrolytic cells to produce hydrogen, which use organic material to produce possible alternative fuels to fossil fuel.
"Fuel cells are able to directly convert fuel into electricity," explains Professor Junhong Chen, the lead researcher. "With the help of fuel cells, it is possible to obtain electrical energy from renewable energy sources when and where it is needed, in a clean, efficient and sustainable manner." The scientists also found that their new catalyst outperformed an alternative graphene-based catalyst under development in other labs. The nanorods have been shown to be stable and commercializable, says the lead researcher, but more experiments are needed to determine how they can be produced in commercial quantities. Further research is also required to determine the exact mechanism responsible for the performance of nanorods. The research findings were published in the scientific journal Advanced Materials.
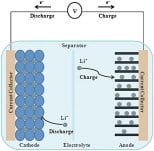
Electricity can be generated from microbial fuel cells while removing contaminants from wastewater. At the anodic electrode of the cell, colonies of bacteria feed on organic matter, while the bacteria emit electrons that are used to generate electricity during the breakdown of the waste. At the cathode, the most important reaction that occurs is the oxygen redox reaction. The platinum catalyst accelerates this slow reaction while increasing the efficiency of the cell's activity, but the catalyst itself is expensive due to the high cost of the platinum metal.
Microbial electrolysis cells are related to microbial fuel cells - in these cells hydrogen is emitted instead of electrons. In addition to utilizing the activity of microorganisms on the side of the anode, these cells also utilize the decomposition of organic matter and platinum in a catalytic process that occurs at the cathode.
The researchers take advantage of the best properties of other active materials when nitrogen is bound to the surface of the carbon rod and iron carbide core. The effectiveness of nitrogen in enhancing the catalytic activity of carbon has long been known. Iron carbide, also known for its catalysis capabilities, reacts with the carbon on the surface of the rod, making contact with the core. In addition, the unique structure of the material has been optimized for the passage of electrons, a mechanism required for cells of this type.
The news about the study
