A rare group of carriers HIV No drugs are needed to control the level of the virus. Their good fortune may pave the way for improved treatments, and perhaps even the development of a vaccine
One day in early 1995, a man named Bob Massey walked into my office in the outpatient department of Massachusetts General Hospital in Boston. Massey told me that 16 years ago he was infected with HIV, the virus that causes AIDS, and had not yet shown any symptoms. The physical examination I performed confirmed that he was healthy, in stark contrast to all the other patients I saw that day. At that time, a new combination of drugs will be tested that will, in the end, slow down the continuous deterioration caused by HIV to the function of the immune system. However, in 1995, most people who had been infected with HIV ten or more years before had already developed AIDS, and were at a stage where their bodies were unable to fight other pathogens. The young man standing in front of me had never taken HIV medication and believed with complete faith that if I deciphered the reasons for his good fortune, I could help others survive what was then considered a fatal disease.
Massey was born with hemophilia, a disease that impairs the clotting ability of the blood. In those days, before the mid or late 80s, almost all hemophiliacs were HIV carriers, because they received repeated transfusions of blood products pooled from thousands of donors, and none of the donors were then tested for HIV. (Nowadays, hemophiliacs receive artificial clotting factors, which carry no risk of infection with the virus.) Some of Massey's blood samples preserved for research purposes showed that he was indeed infected with HIV in 1978. However, every test I conducted on him or on these blood samples showed that the amount of virus in his blood was minimal and that his immune system activity was as strong as ever.
I was stunned. This was the first time I encountered a patient whose body manages to control HIV on its own, and he has been doing so for about 15 years. But it turned out that Massey is not one of a kind. Researchers in California, Maryland, Italy and France also encountered such virtuous individuals in the early 90s and studied them diligently. In the end, we determined that these unique people split into two main groups: one of "long-term stable" people, whose bodies were able to fight HIV for a considerable time, but eventually got sick. And the other group, much smaller, was even more amazing and included people with "elite control" of HIV. These people, like Massey, simply did not develop AIDS, year after year after year, even though they never took HIV drugs.
Somehow, the elite control people managed to keep extremely low, sometimes undetectable, levels of the virus in their blood. If scientists manage to understand how these rare people manage to do so, they may understand how to create an effective ingredient or develop drugs that will strengthen the immune system of patients, and not just attack the virus with drugs. And time is running out. Today, approximately 33 million people live with HIV worldwide. Drug treatment is available to more than 6 million of them, but these drugs are unable to cure HIV infection, and must be taken for life. There are only slim chances that the drug treatment will be given to everyone who needs it as long as they need it. We are urgently looking for a solution that will prevent infection in those who have not yet been infected and prevent the disease from developing in those who have already been infected.
After two decades of studying elite control people like Massey, my colleagues and I are more convinced than ever that the unique biochemical properties of these people provide outstanding insights into the prevention and treatment of AIDS. This scientific odyssey has far-reaching implications for other human infectious diseases and possibly even some cancers.
There are not enough generals
To understand how unusual Massey and other elite-controlled people are and why they inspire hope for the eradication of AIDS and other diseases, it is useful to first understand how HIV attacks the body and how the body tries to defend itself against it. Over the past 30 years, researchers have learned that the immune system of most people who become infected with HIV, and not just those of the elite, puts up a fierce fight against the initial infection, and produces a large amount of antibodies against the virus. Unfortunately, the antibodies are ineffective, and because of this the virus remains in the body, even in the bodies of those with elite control. The exact mechanisms that confer control, without good antibodies, are quite complex and sometimes even mysterious. But the bottom line is that two different types of immune cells, called helper T cells (or CD4+ cells) and killer T cells (or CD8+ cells), and molecules called HLA receptors, play the most important roles.
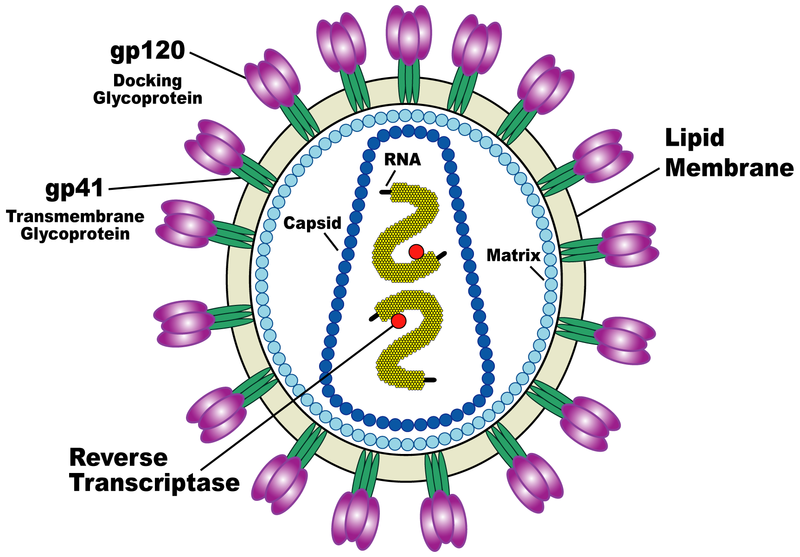
Like any virus, HIV is also unable to reproduce on its own. When it infects cells, it takes over their internal mechanisms and causes them to produce new viruses instead of fulfilling the normal cellular functions. However, the infected cells have a warning system that warns the body against the invaders. In the first hours of a viral invasion, the infected cells transport to their surface pieces of virus proteins that they were forced to produce. These pieces of foreign material are presented to the body, on the surface of the cells, with the help of the HLA receptors. The presence of viral proteins conjugated to the HLA molecules of these cells soon attracts the attention of the immune system. As a result, the helper T cells are activated and mobilize a group of killer T cells that are now programmed to destroy HIV-infected cells. The activated helper T cells also gradually cause other cells of the immune system to produce antibodies that attach to certain components of the viruses released from the cells, in a separate, if fruitless, effort to eliminate the invaders.
This defense system works quite effectively against most viral infections. But HIV performs an unusual trick that ultimately defeats the immune system: the virus preferentially infects the helper T cells, including the cells that were activated to help protect against it. This viral damaging action leads directly or indirectly to the destruction of most of the available helper T cells. If you compare the T-cells that help the generals of the immune system and the T-cells that kill the ordinary soldiers, then HIV harms the accuracy of a sniper in the generals, and harms their ability to give the soldiers effective instructions regarding the continuation of the battle. In the simplest sense, HIV is an infection of the immune system, and the results are predictable: destruction of the body's ability to protect itself not only from HIV but also from hundreds of other invaders.
When Bob Massey appeared in my office in the mid-90s, my lab was focused on the role of killer T cells in the fight against HIV. We hypothesized that if Massey's immune system is indeed controlling the level of the virus, the killer T-cell response in his body is particularly strong. We incorporated it into the research we did, and we soon realized that the killer T-cell response in his body against HIV was the strongest response we had ever seen. In other words, his immune system produced a large infantry specially trained to recognize HIV. This result was consistent with our hypothesis, but even carriers and other HIV carriers occasionally had a strong killer T-cell response, yet they still developed AIDS. That is to say, there are cases where the infantry does have large numbers of soldiers, yet is unable to fight effectively.
This observation, in turn, led to a second hypothesis. Massey's killer T cells may have been particularly effective because they received appropriate instructions from particularly effective helper T cells. In other words, both his generals (helper T cells) and foot soldiers (killer T cells) were impressively trained.
The case ran and the first project I undertook when I began my research career in the mid-80s looked at the specific steps by which helper T cells orchestrate the immune system to attack HIV. My colleagues and I tested blood samples from dozens of AIDS patients for evidence that helper T cells coordinate a counterattack. But we found nothing even after many months of trying. It was almost as if the immune system was unable to produce such a high level response. Indeed, the absence of HIV-specific helper T cells was the most prominent deficiency in the defense system of people infected with HIV.
But Bob didn't get AIDS. He successfully controlled the viral infection. That's why we dusted off the experimental system that I used ten years before. This time we saw exactly what we expected to see in a case where the immune system does control the infection: not only did Massey have the same generals trained to mount an attack against HIV, but he also had massive amounts of them. We published our findings in the journal Science in 1997. Our paper showed that helper T cells from people infected with HIV can sometimes respond effectively against the virus, a finding that fundamentally changed the way our research group thought about the virus. It will finally be seen that the immune system can, in some cases, beat the virus that has killed millions of people around the world.
Other questions
As with many scientific discoveries, our finding that an effective killer T-cell response against HIV depends on a strong team of helper T cells has raised many additional questions and hypotheses. Did Massey really clear the virus from his body? The answer was no, because we could detect genetic material of the virus in his blood. [To understand why some people, unlike Massey, are actually immune to HIV, see "No Entry to HIV", by Carl John and Bruce Levine, Scientific American Israel, June 2012]. Can Massey infect others? We didn't know the answer but we had to assume it was positive, something important for him and his wife (they eventually had a daughter). Was his immune system somehow a super-system capable of fighting any possible intruder? The answer here, unfortunately, was no, because he also suffered from a hepatitis C virus infection, another result of receiving contaminated blood products as a treatment for hemophilia, and his body was unable to control this infection at all. (Massey eventually underwent a liver transplant, which cured his jaundice, and because the new liver could produce the necessary clotting factors, the transplant also cured his hemophilia.)
We considered the possibility that each infected person does, in fact, produce HIV-specific helper T cells, but these trained generals are killed at very early stages after the initial invasion. But if that were the case, aggressive treatment against the virus with a powerful drug cocktail that would completely inhibit virus production should protect the helper T cells of newly infected people. Such a strong first blow should allow the immune system to gain a quick advantage over the virus and maintain this advantage effectively, as happened naturally with Bob. We performed clinical trials on several dozen volunteers and showed that early treatment lowered the level of the virus in the blood quickly below the sensitivity threshold of the tests, and within a few weeks a massive production of helper T cells was made possible that are capable of instructing killer T cells to fight HIV. In other words, most people's immune systems are able to produce competent generalists (helper T cells unique to HIV), but they are eliminated almost as soon as they are produced.
Unfortunately, the newly created defense did not provide the ability to control the level of the virus over time as we saw with Massey. Later in the clinical trial, we stopped the treatment of a handful of patients (who gave their informed consent and after receiving approval from the ethics committee). The subjects remained without treatment for a year or more, and most of them experienced a gradual increase in the level of the virus in their blood, so that in the end they had to return to the drug cocktail. Nevertheless, the results, published in the journal Nature in 2000, showed that it is possible to increase, at least temporarily, the body's level of control of HIV. Moreover, it is possible to activate in other people the same mechanism that allowed Massey to control his infection.
How is it possible to induce such a level of immune control over time, similar to people with elite control? So far we have looked at the same immune responses that we know how to measure, namely helper T cells and killer T cells. We had to delve deeper into the intricacies of the immune system to understand, once and for all, what the difference is that provides high control people with protection from the horrors of HIV.
Credit: Wikipedia
A new approach
A deeper understanding of the basis of HIV control was made possible by a series of discounted sessions. Around that time I was invited to dinner with Lawrence Summers, who was then president of Harvard University, to discuss the growing involvement of the university's medical school in global health. Another guest was Eric Lander, a former classmate of Massey's at Princeton University, and an expert in applying the latest findings in human genetics to medical research. Before that, I had not had the opportunity to meet Lander, who directed the then established Broad Institute, a joint venture between Harvard and the Massachusetts Institute of Technology (MIT), but I had wanted to meet him for some time, because it seemed that the new technology he had developed would provide us with insights into the problem of -HIV.
Our mutual acquaintance with Massey was the starting point for an ongoing discussion that night on the sidewalk outside Summers' house. Lander explained that it is possible to compare the DNA of different people, by looking for natural variation in the letters A, T, C and G in the DNA sequence. These differences, called SNPs, make it possible to identify genetic influences on the individual's response to a given disease. The SNPs will be used as markers to point to areas in the genome of high-control individuals like Massey that allow them to minimize the damage caused by HIV. If we can find a unique pattern of SNPs associated with controlling the virus, then this pattern may help us locate the responsible genes, if they exist. To conduct the research, it is necessary to obtain a saliva or blood sample from people with high control of the virus and HIV carriers who have had AIDS, and then extract DNA from these samples. At the very least, we would have to examine about a million SNPs from each of 1,000 people with elite control, and about twice as many AIDS patients to get statistically adequate results.
Extracting DNA from a large number of people with AIDS must not have been difficult. But the insurmountable obstacle was finding a large number of people with elite control. Until then we and other researchers around the world knew only a handful of such rare individuals, and finding 1,000 elite controlled individuals seemed an impossible task.
Around the same time, I was invited to give a lecture in New York City to 300 medical professionals who ran large AIDS clinics. My mission was to inform the doctors about how HIV causes AIDS. During the lecture I mentioned the case of Massey, who had been a carrier for more than a quarter of a century at the time, had never been treated, and still had a normal helper T-cell count and undetectable amounts of virus in his blood. (Right then, HIV tests became much more sensitive and could detect even 50 copies of the virus in a milliliter of blood. And Massey always had a number lower than that.) In a moment's decision, I asked the audience that anyone who encountered a similar case would raise their hand.
I must have read an exclamation when more than half the people in the hall raised their hands. Here is the solution to the problem of finding 1,000 people with elite control! With the help of the medical staff in this hall alone, we can reach perhaps even 200 such extraordinary people. If we can directly contact doctors and nurses in private clinics across the country and ask them to refer us to people with high control, we can easily reach the number required to conduct a statistically significant study that will determine whether there are specific genetic variants that increase or impair the immune system's ability to fight HIV.
Massachusetts General Hospital (MGH) approved us to pursue such a study. But we soon encountered another obstacle. We submitted requests for funding from several organizations but were denied. They thought that our goals were too vague because we didn't know what exactly we were looking for and the chances of success seemed to them to be minimal.
While we were struggling with this disappointing delay, Mark Schwartz, former chairman of Goldman Sachs Bank (Asia), invited me to breakfast at a hotel in New York. Schwartz and his wife, Lisa, began funding some of the MGH and Harvard programs to train scientists and doctors in Africa to help overcome the AIDS crisis. During our meeting, Schwartz asked me what else I was working on. While answering, I expressed my frustration with the Elite Controlled People project and stated that in my view it would yield vital information that would lead us forward. Schwartz immediately straightened up when I explained the logic behind the study. Why wouldn't he and his wife fund the research, he asked. To my astonishment, by the end of the meeting the Schwartzes had committed to donating $2.5 million over the next five years to launch our research on people with elite control of the virus. The budget will be used to recruit patients from across the country, and the successful recruitment of subjects will help convince other funders to pay for the genetic analysis.
We immediately started the research, contacting all the main HIV therapists across the US. Over time we collected DNA samples from patients in Europe, Asia, Australia and South America. We tried to include people with elite control from Africa, but we had difficulty locating them because in many countries in Africa they did not perform tests of the level of the virus in the blood as a routine. Florencia Ferreira, a physician-scientist at Harvard Medical School, organized the massive recruiting operation with the help of one assistant, then two and three. The Bill and Melinda Gates Foundation provided us with a $20 million grant over five years to complete the research.
Processing and analyzing the data took almost as long as collecting the samples. For each of the 974 elite controls and 2,684 AIDS patients in our study, we tested 1.3 million SNPs in DNA using an automated DNA microarray system. We relied on the powerful computing services of the Broad Institute to compare the SNPs of the two groups. Paul De Becker, a geneticist at the institute, conducted the computerized analysis.
In 2009 we received a preliminary answer. Of the three billion nucleotides (or letters) in the human genome, 300 SNPs were statistically significantly different in people with elite control versus people prone to AIDS. Further analyzes diluted the 300 SNPs to only four, each of which independently found a high correlation with control of the viral infection. All four are found on chromosome 6, which contains many genes that affect the immune system. But we still didn't know which gene, or which genes, were important and why.
But at least we now knew where to look. The next step was to determine the complete genetic sequence of the region on chromosome 6 where the SNPs were concentrated. Although we did not have funding to perform this additional and detailed sequencing, Xiaoming "Sherman" Jia, an extraordinary medical student, solved the problem for us. He used vast databases from other genetic studies and developed a computer program that used the combinations of each person's SNPs to deduce the exact sequence of nucleotides, or DNA code letters, in that particular region of chromosome 6. With this sequence it was possible to deduce the sequence of amino acids in the protein encoded by the DNA in that region.
As happens when you go to a higher magnification in a microscope, Sherman's software suddenly sharpened the image. The main genetic difference between people with high HIV control and people who got sick was a change in amino acids that affected the shape of a depression in the HLA receptors found on the surface of infected cells. Within this particular recess are held pieces of HIV proteins that the HLA receptors present to other cells. In people with elite control, something about the recess made the binding between HLA and HIV proteins on infected cells particularly attractive to killer T cells, which consequently destroyed the infected cells. It is similar to a worker in a factory who wants to warn the environment that bomb-making terrorists have taken over the factory. The worker paints his hands and a piece of bomb bright orange and waves them through the window to attract the attention of passers-by. Doing so helps the authorities notice that something bad is going on, so they can come and deal with the threat.
Finally a hidden piece of the attachment was found and the reason why Massey and other elite controlled people remain healthy after so many years. From the first days of infection, their immune system maintains a critical number of healthy helper T cells unique to HIV. These cells provide vital instructions to killer T cells that undergo activation. These soldiers of the immune system are in turn able to effectively find and destroy HIV-infected cells because the HLA molecules on these cells, which are doomed to the kidney, are genetically programmed to alert the killer T cells of the invader's presence better than the HLA molecules of most the people.
Consequently, by maintaining a low level of virus, these effective killer T cells protect the helper T cells from infection. The infantry guards the generals, allowing the immune system to fight until the virus is completely stopped. The low-probability genetic approach we took, which began with no definite hypothesis and relied on the collaboration of more than 300 researchers worldwide, revealed that the primary genetic basis for long-term HIV control lies in the properties of a single protein, the HLA molecule.
And again, the findings, published in Science in 2010, raised new questions. We need to understand how to create such an immune response in most infected people. Also, we are beginning to understand what is involved in trying to adjust the body's defense systems against specific diseases. First and foremost it is necessary to increase the appropriate activity of helper and killer T cells. [To read more about T-cell cancer therapy, see "A New Ally Against Cancer" by Eric von Hoff, Scientific American Israel, February-March 2012]. The immune system has always been an imperfect partner in our fight against disease. We have much more to learn, but we hope that soon we will be able to help her fill in the gaps.
______________________________________________________________________________________________________________________________________________________________________________________
About the author
Bruce D. Walker (Walker) saw his first AIDS patient in 1981, when he was still an intern. He is currently the director of the Regon Institute in Boston, a professor of medicine at Harvard Medical School, and an associate professor at the University of KwaZulu-Natal in Durban, South Africa.
And more on the subject
The Major Genetic Determinants of HIV-1 Control Affect HLA Class I Peptide Presentation. International HIV Controllers Study in Science, Vol. 330, pages 1551–1557; December 10, 2010. www.ncbi.nlm.nih.gov/pmc/articles/PMC3235490
Immunogenetics of Spontaneous Control of HIV. Mary Carrington and Bruce D. Walker in Annual Review of Medicine, Vol. 63, pages 131–145; February 2012. www.ncbi.nlm.nih.gov/pubmed/22248321
A Song in the Night: A Memoir of Resilience. Bob Massie. Nan A. Talese, 2012.




5 תגובות
An interesting article by all accounts and from here, at least in my estimation, it is possible to project another super-resistance to a similar viral disease. The virus HTLV 1/2, or in its full name - Human T-lymphotropic virus type 1/2 is a retrovirus, similar to HIV, which also infects CD4+ cells (helper T cells). The striking difference between the two caused diseases is that the HTLV virus does not cause such a massive destruction of helper T cells in a short period of time and after about two decades (20-25 years from the moment of infection) may lead to various blood cancers, such as leukemia.
A second comment that I couldn't help but think about is the complete lack of reference to an additional player, you want another soldier in the infantry of the immune system, aka the natural killer cell. These cells (NKs) are another important factor, along with CD8+ cells (killer T cells), in dealing with pathogens, including the HIV virus.
Many viruses have developed mechanisms to prevent the expression of the cellular HLA molecule (including the HIV virus) in order to prevent the elimination of the infected cells by the deadly T cells. This is exactly where the NK comes into play. Its function is to monitor cells without the HLA molecule and to destroy these cells.
In conclusion, there is no doubt that the role of the killer T cells in dealing with HIV infection is critical, but the NK cells should not be ignored. It is interesting to test the role of SNPs in the expression of functional elements in NK cells in this infection.
Thanks Muti!
Here is the requested link:
http://sciam.co.il/archives/5324
I will check after the holiday with Scientific. If you find it on their site please send a link.
Hello my father,
I would like, if you could, to upload to the site the article "There is no entry to HIV", by Carl John and Bruce Levin, Scientific American Israel, June 2012, which is mentioned in the current article and in my opinion the public browsing the site will be able to learn a lot more about the subject from it.
Thanks in advance.